Abstract
BACKGROUND—Inactivation of the tumour suppressor gene p16 (CDKN2/MTS-1/INK4A) and K-ras mutations are among the most frequent genetic alterations in human malignancies. AIMS—To investigate the tumour suppressor gene p16 and its possible association with K-ras mutations in intrahepatic cholangiocarcinomas of the liver. METHODS—The status of p16 was evaluated in 41 cholangiocarcinomas by methylation specific polymerase chain reaction, microsatellite analysis, DNA sequencing, and immunohistochemical staining. K-ras mutations were determined by direct DNA sequencing analyses after microdissection. The results obtained were correlated with histopathological variables and patient survival. RESULTS—Hypermethylation of the 5' CpG island of the p16 gene was found in 34 of 41 (83%) carcinomas. Homozygous deletion at the p16 region was present in two (5%), and loss of heterozygosity (LOH) in eight cases (20%). We failed to detect p16 gene missense mutations. K-ras mutations were found in 22 of 41 (54%) cholangiocarcinomas and in two cases of tumour surrounding non-neoplastic liver tissue. All 22 cancers with K-ras mutations also exhibited methylated p16. We failed to observe a correlation between K-ras or p16 status and histopathological factors or prognosis of patients. CONCLUSION—These data suggest that inactivation of the p16 gene is a frequent event in cholangiocarcinoma. The most common somatic alteration is promotor methylation of the p16 gene which is closely associated with K-ras mutations. We failed to establish p16 or K-ras status as independent prognostic factors in these tumours. Keywords: cholangiocarcinoma; p16; K-ras; histopathology; prognosis; methylation
Full Text
The Full Text of this article is available as a PDF (166.0 KB).
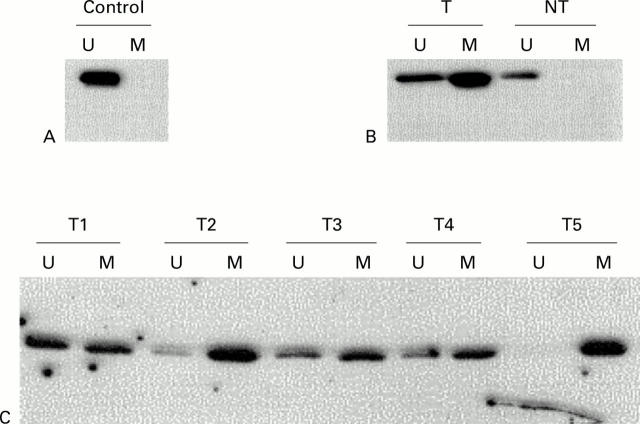
Figure 1 .
Methylation analysis of p16 in cholangiocarcinoma. Methylation specific PCR (MSP) results are expressed as unmethylated p16 specific bands (U) or methylated p16 specific bands (M). (A) Bisulphite converted DNA from normal liver tissue (N) served as a negative control as indicated by the presence of the U but not the M band. (B) MSP results of case No 28. The tumour surrounding non-neoplastic liver tissue (NT) with unmethylated p16. (C) Representative cases of cholangiocarcinoma. The numbers of the cholangiocarcinoma cases are shown above the corresponding lanes.
Figure 2 .
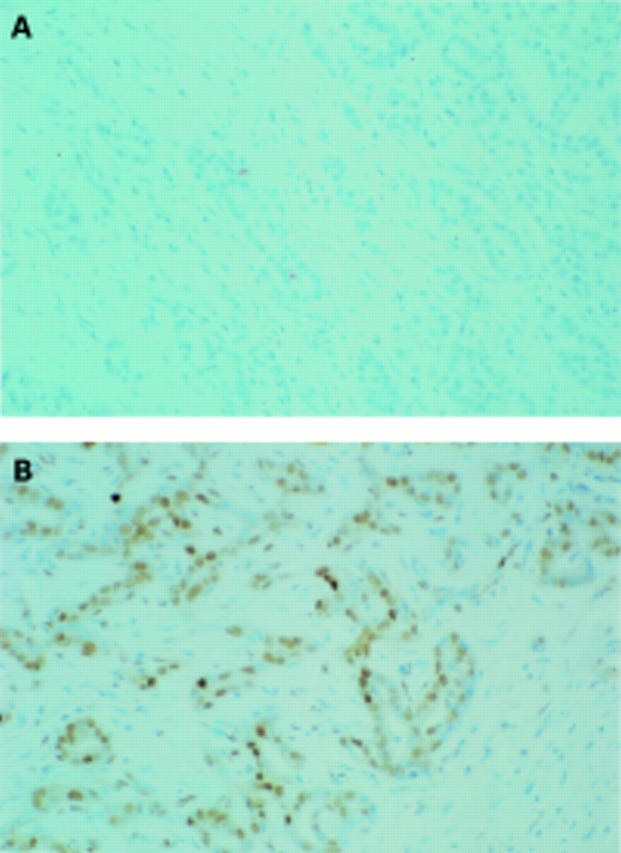
Immunostaining of p16 protein in cholangiocarcinoma. (A) Patient No 28 (same patient as in figure 1B) with a methylated p16 gene. Complete loss of p16 expression. (B) Immunohistochemical staining of p16 in a moderately differentiated cholangiocarcinoma without p16 methylation. Strong nuclear positivity of the tumour cells (brown reaction product). (Original magnification ×40.)
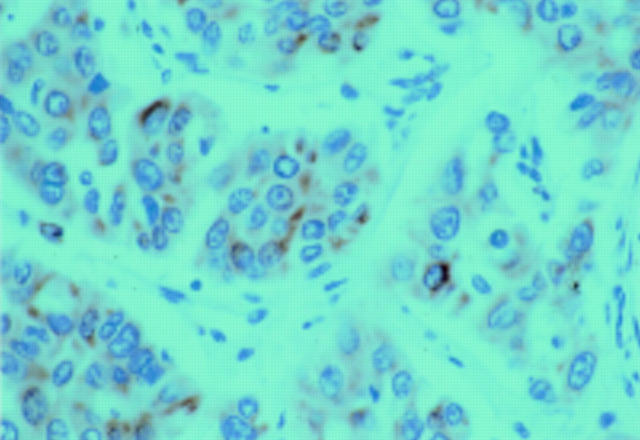
Figure 3 .
Immunostaining of the ras protein with perinuclear staining (red reaction product) of tumour cells. (Original magnification ×60.)
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahrendt S. A., Eisenberger C. F., Yip L., Rashid A., Chow J. T., Pitt H. A., Sidransky D. Chromosome 9p21 loss and p16 inactivation in primary sclerosing cholangitis-associated cholangiocarcinoma. J Surg Res. 1999 Jun 1;84(1):88–93. doi: 10.1006/jsre.1999.5615. [DOI] [PubMed] [Google Scholar]
- Andreyev H. J., Tilsed J. V., Cunningham D., Sampson S. A., Norman A. R., Schneider H. J., Clarke P. A. K-ras mutations in patients with early colorectal cancers. Gut. 1997 Sep;41(3):323–329. doi: 10.1136/gut.41.3.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjørheim J., Lystad S., Lindblom A., Kressner U., Westring S., Wahlberg S., Lindmark G., Gaudernack G., Ekstrøm P., Røe J. Mutation analyses of KRAS exon 1 comparing three different techniques: temporal temperature gradient electrophoresis, constant denaturant capillary electrophoresis and allele specific polymerase chain reaction. Mutat Res. 1998 Jul 17;403(1-2):103–112. doi: 10.1016/s0027-5107(98)00057-8. [DOI] [PubMed] [Google Scholar]
- Chaubert P., Gayer R., Zimmermann A., Fontolliet C., Stamm B., Bosman F., Shaw P. Germ-line mutations of the p16INK4(MTS1) gene occur in a subset of patients with hepatocellular carcinoma. Hepatology. 1997 Jun;25(6):1376–1381. doi: 10.1002/hep.510250613. [DOI] [PubMed] [Google Scholar]
- Deng C., Yang J., Scott J., Hanash S., Richardson B. C. Role of the ras-MAPK signaling pathway in the DNA methyltransferase response to DNA hypomethylation. Biol Chem. 1998 Aug-Sep;379(8-9):1113–1120. doi: 10.1515/bchm.1998.379.8-9.1113. [DOI] [PubMed] [Google Scholar]
- Dogliotti E., Hainaut P., Hernandez T., D'Errico M., DeMarini D. M. Mutation spectra resulting from carcinogenic exposure: from model systems to cancer-related genes. Recent Results Cancer Res. 1998;154:97–124. doi: 10.1007/978-3-642-46870-4_6. [DOI] [PubMed] [Google Scholar]
- Franco M., Chardin P., Chabre M., Paris S. Myristoylation of ADP-ribosylation factor 1 facilitates nucleotide exchange at physiological Mg2+ levels. J Biol Chem. 1995 Jan 20;270(3):1337–1341. doi: 10.1074/jbc.270.3.1337. [DOI] [PubMed] [Google Scholar]
- Guan R. J., Fu Y., Holt P. R., Pardee A. B. Association of K-ras mutations with p16 methylation in human colon cancer. Gastroenterology. 1999 May;116(5):1063–1071. doi: 10.1016/s0016-5085(99)70009-0. [DOI] [PubMed] [Google Scholar]
- Hussussian C. J., Struewing J. P., Goldstein A. M., Higgins P. A., Ally D. S., Sheahan M. D., Clark W. H., Jr, Tucker M. A., Dracopoli N. C. Germline p16 mutations in familial melanoma. Nat Genet. 1994 Sep;8(1):15–21. doi: 10.1038/ng0994-15. [DOI] [PubMed] [Google Scholar]
- Kiba T., Tsuda H., Pairojkul C., Inoue S., Sugimura T., Hirohashi S. Mutations of the p53 tumor suppressor gene and the ras gene family in intrahepatic cholangiocellular carcinomas in Japan and Thailand. Mol Carcinog. 1993;8(4):312–318. doi: 10.1002/mc.2940080415. [DOI] [PubMed] [Google Scholar]
- Kjoller L., Hall A. Signaling to Rho GTPases. Exp Cell Res. 1999 Nov 25;253(1):166–179. doi: 10.1006/excr.1999.4674. [DOI] [PubMed] [Google Scholar]
- Liew C. T., Li H. M., Lo K. W., Leow C. K., Chan J. Y., Hin L. Y., Lau W. Y., Lai P. B., Lim B. K., Huang J. High frequency of p16INK4A gene alterations in hepatocellular carcinoma. Oncogene. 1999 Jan 21;18(3):789–795. doi: 10.1038/sj.onc.1202359. [DOI] [PubMed] [Google Scholar]
- Matsuda Y., Ichida T., Matsuzawa J., Sugimura K., Asakura H. p16(INK4) is inactivated by extensive CpG methylation in human hepatocellular carcinoma. Gastroenterology. 1999 Feb;116(2):394–400. doi: 10.1016/s0016-5085(99)70137-x. [DOI] [PubMed] [Google Scholar]
- Morrin M., Kelly M., Barrett N., Delaney P. Mutations of Ki-ras and p53 genes in colorectal cancer and their prognostic significance. Gut. 1994 Nov;35(11):1627–1631. doi: 10.1136/gut.35.11.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigam A. K., Pignatelli M., Boulos P. B. Current concepts in metastasis. Gut. 1994 Jul;35(7):996–1000. doi: 10.1136/gut.35.7.996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantoja C., Serrano M. Murine fibroblasts lacking p21 undergo senescence and are resistant to transformation by oncogenic Ras. Oncogene. 1999 Sep 2;18(35):4974–4982. doi: 10.1038/sj.onc.1202880. [DOI] [PubMed] [Google Scholar]
- Rashid A., Zahurak M., Goodman S. N., Hamilton S. R. Genetic epidemiology of mutated K-ras proto-oncogene, altered suppressor genes, and microsatellite instability in colorectal adenomas. Gut. 1999 Jun;44(6):826–833. doi: 10.1136/gut.44.6.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzi P. M., Ryder S. D., Portmann B., Ramage J. K., Naoumov N. V., Williams R. p53 Protein overexpression in cholangiocarcinoma arising in primary sclerosing cholangitis. Gut. 1996 Feb;38(2):265–268. doi: 10.1136/gut.38.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussel M. F. The INK4 family of cell cycle inhibitors in cancer. Oncogene. 1999 Sep 20;18(38):5311–5317. doi: 10.1038/sj.onc.1202998. [DOI] [PubMed] [Google Scholar]
- Serrano M., Gómez-Lahoz E., DePinho R. A., Beach D., Bar-Sagi D. Inhibition of ras-induced proliferation and cellular transformation by p16INK4. Science. 1995 Jan 13;267(5195):249–252. doi: 10.1126/science.7809631. [DOI] [PubMed] [Google Scholar]
- Serrano M., Lee H., Chin L., Cordon-Cardo C., Beach D., DePinho R. A. Role of the INK4a locus in tumor suppression and cell mortality. Cell. 1996 Apr 5;85(1):27–37. doi: 10.1016/s0092-8674(00)81079-x. [DOI] [PubMed] [Google Scholar]
- Serrano M., Lin A. W., McCurrach M. E., Beach D., Lowe S. W. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell. 1997 Mar 7;88(5):593–602. doi: 10.1016/s0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]
- Soman N. R., Wogan G. N. Activation of the c-Ki-ras oncogene in aflatoxin B1-induced hepatocellular carcinoma and adenoma in the rat: detection by denaturing gradient gel electrophoresis. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):2045–2049. doi: 10.1073/pnas.90.5.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannapfel A., Engeland K., Weinans L., Katalinic A., Hauss J., Mössner J., Wittekind C. Expression of p73, a novel protein related to the p53 tumour suppressor p53, and apoptosis in cholangiocellular carcinoma of the liver. Br J Cancer. 1999 Jun;80(7):1069–1074. doi: 10.1038/sj.bjc.6690465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannapfel A., Wasner M., Krause K., Geissler F., Katalinic A., Hauss J., Mössner J., Engeland K., Wittekind C. Expression of p73 and its relation to histopathology and prognosis in hepatocellular carcinoma. J Natl Cancer Inst. 1999 Jul 7;91(13):1154–1158. doi: 10.1093/jnci/91.13.1154. [DOI] [PubMed] [Google Scholar]
- Wei S., Wei S., Sedivy J. M. Expression of catalytically active telomerase does not prevent premature senescence caused by overexpression of oncogenic Ha-Ras in normal human fibroblasts. Cancer Res. 1999 Apr 1;59(7):1539–1543. [PubMed] [Google Scholar]