Abstract
BACKGROUND AND AIMS—The mechanism of gastrointestinal damage (mucositis) induced by cancer chemotherapy remains uncertain. The aims of this study were to define the time course and mechanism of small intestinal damage following chemotherapy in humans. METHODS—Patients receiving chemotherapy underwent upper gastrointestinal endoscopy (a maximum of two per patient) with duodenal biopsy prior to chemotherapy and again at 1, 3, 5, and 16 days after chemotherapy. Tissue was taken for morphometry, disaccharidase assays, electron microscopy, and for assessment of apoptosis using the Tdt mediated dUTP-biotin nick end labelling (TUNEL) method. Villus area, crypt length, and mitotic index were measured by a microdissection technique. RESULTS—Apoptosis increased sevenfold in intestinal crypts at one day, and villus area, crypt length, mitotic count per crypt, and enterocyte height decreased at three days after chemotherapy. Disaccharidase activities remained unchanged. Electron microscopy showed increased open tight junctions of enterocytes at day 3, consistent with more immature cells. All indices improved by 16 days. CONCLUSION—Small intestinal mucositis is associated with apoptosis in crypts that precedes hypoplastic villous atrophy and loss of enterocyte height. Keywords: chemotherapy; mucositis; small intestine
Full Text
The Full Text of this article is available as a PDF (158.4 KB).
Figure 1 .
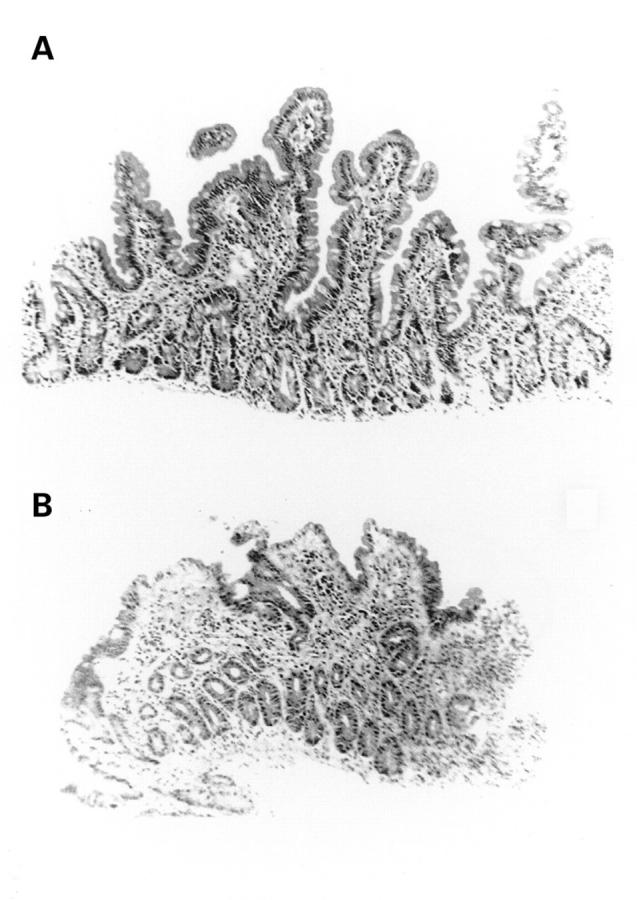
Changes in intestinal morphology before (A) and at three days after (B) chemotherapy for cancer. (Original magnification ×156.)
Figure 2 .
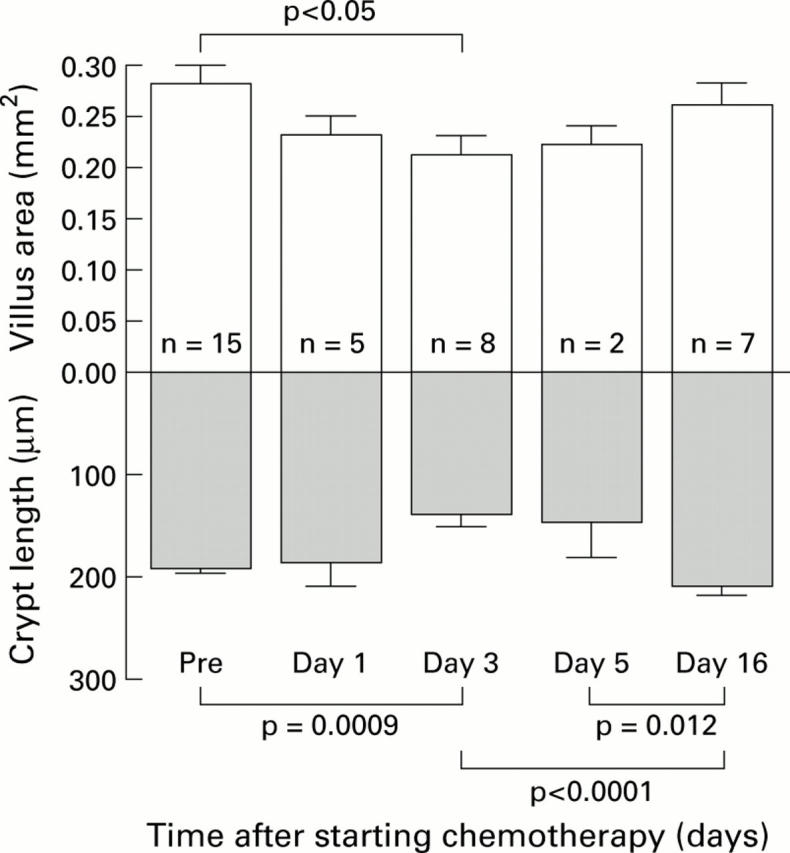
Changes in villus area and crypt length before (Pre) and after chemotherapy for cancer. Data are given as mean (SEM).
Figure 3 .
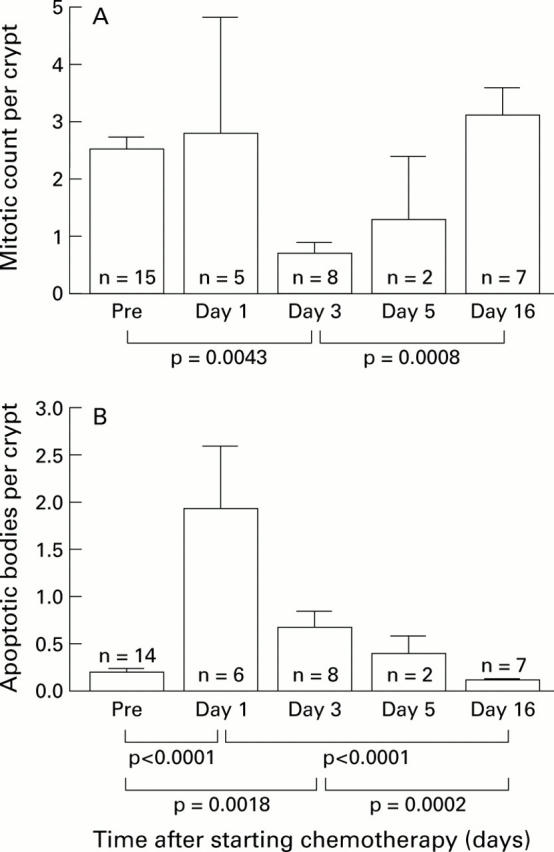
Changes in mitotic count per crypt (A) and apoptotic count per crypt per 4 µm section (B) before (Pre) and after chemotherapy for cancer. Data are given as mean (SEM).
Figure 4 .
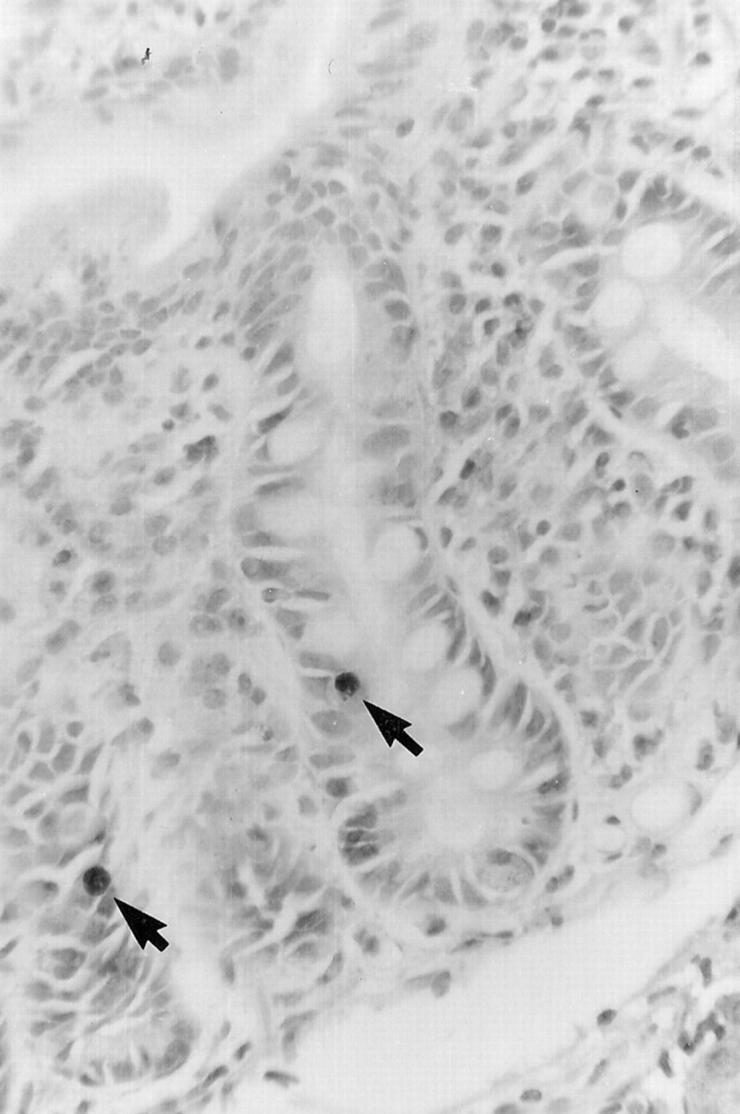
Light photomicrograph showing two apoptotic cells (arrows) in the crypt of the small intestine at one day after chemotherapy. Apoptosis was detected using the TUNEL technique. (Original magnification ×292.)
Figure 5 .
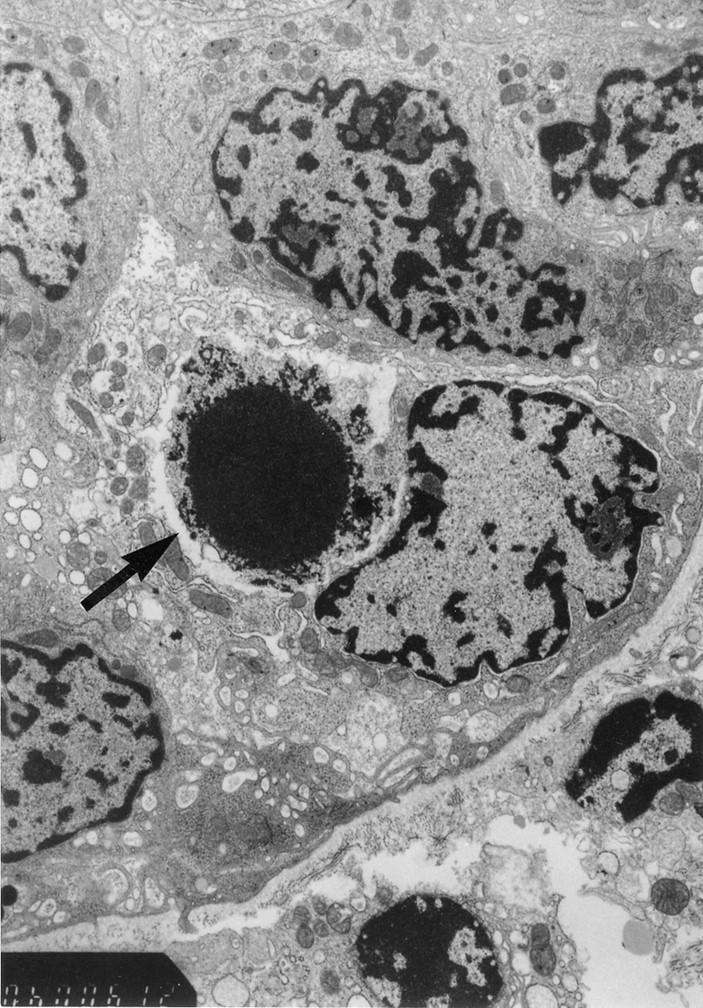
Electron micrograph of an apoptotic cell in the epithelial layer of the small intestinal crypt at one day after chemotherapy. The arrow indicates an apoptotic cell with a pyknotic nucleus. (Original magnification ×12 000.)
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cummins A. G., LaBrooy J. T., Stanley D. P., Rowland R., Shearman D. J. Quantitative histological study of enteropathy associated with HIV infection. Gut. 1990 Mar;31(3):317–321. doi: 10.1136/gut.31.3.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham D., Morgan R. J., Mills P. R., Nelson L. M., Toner P. G., Soukop M., McArdle C. S., Russell R. I. Functional and structural changes of the human proximal small intestine after cytotoxic therapy. J Clin Pathol. 1985 Mar;38(3):265–270. doi: 10.1136/jcp.38.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrieli Y., Sherman Y., Ben-Sasson S. A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992 Nov;119(3):493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannun Y. A. Apoptosis and the dilemma of cancer chemotherapy. Blood. 1997 Mar 15;89(6):1845–1853. [PubMed] [Google Scholar]
- Harper J. F. Peritz' F test: basic program of a robust multiple comparison test for statistical analysis of all differences among group means. Comput Biol Med. 1984;14(4):437–445. doi: 10.1016/0010-4825(84)90044-1. [DOI] [PubMed] [Google Scholar]
- Ijiri K., Potten C. S. Further studies on the response of intestinal crypt cells of different hierarchical status to eighteen different cytotoxic agents. Br J Cancer. 1987 Feb;55(2):113–123. doi: 10.1038/bjc.1987.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ijiri K., Potten C. S. Response of intestinal cells of differing topographical and hierarchical status to ten cytotoxic drugs and five sources of radiation. Br J Cancer. 1983 Feb;47(2):175–185. doi: 10.1038/bjc.1983.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeynes B. J., Altmann G. G. Light and scanning electron microscopic observations of the effects of sublethal doses of methotrexate on the rat small intestine. Anat Rec. 1978 May;191(1):1–17. doi: 10.1002/ar.1091910102. [DOI] [PubMed] [Google Scholar]
- Keefe D. M., Cummins A. G., Dale B. M., Kotasek D., Robb T. A., Sage R. E. Effect of high-dose chemotherapy on intestinal permeability in humans. Clin Sci (Lond) 1997 Apr;92(4):385–389. doi: 10.1042/cs0920385. [DOI] [PubMed] [Google Scholar]
- Langman J. M., Rowland R. Activity of duodenal disaccharidases in relation to normal and abnormal mucosal morphology. J Clin Pathol. 1990 Jul;43(7):537–540. doi: 10.1136/jcp.43.7.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell E. P., Schein P. S. Gastrointestinal toxicity of chemotherapeutic agents. Semin Oncol. 1982 Mar;9(1):52–64. [PubMed] [Google Scholar]
- Potten C. S., Wilson J. W., Booth C. Regulation and significance of apoptosis in the stem cells of the gastrointestinal epithelium. Stem Cells. 1997;15(2):82–93. doi: 10.1002/stem.150082. [DOI] [PubMed] [Google Scholar]
- Potten C. S., Wilson J. W., Booth C. Regulation and significance of apoptosis in the stem cells of the gastrointestinal epithelium. Stem Cells. 1997;15(2):82–93. doi: 10.1002/stem.150082. [DOI] [PubMed] [Google Scholar]
- Pritchard D. M., Potten C. S., Hickman J. A. The relationships between p53-dependent apoptosis, inhibition of proliferation, and 5-fluorouracil-induced histopathology in murine intestinal epithelia. Cancer Res. 1998 Dec 1;58(23):5453–5465. [PubMed] [Google Scholar]
- Searle J., Lawson T. A., Abbott P. J., Harmon B., Kerr J. F. An electron-microscope study of the mode of cell death induced by cancer-chemotherapeutic agents in populations of proliferating normal and neoplastic cells. J Pathol. 1975 Jul;116(3):129–138. doi: 10.1002/path.1711160302. [DOI] [PubMed] [Google Scholar]
- Smith F. P., Kisner D. L., Widerlite L., Schein P. S. Chemotherapeutic alteration of small intestinal morphology and function: a progress report. J Clin Gastroenterol. 1979 Sep;1(3):203–207. doi: 10.1097/00004836-197909000-00003. [DOI] [PubMed] [Google Scholar]
- TRIER J. S. Morphologic alterations induced by methotrexate in the mucosa of human proximal intestine. II. Electron microscopic observations. Gastroenterology. 1962 Oct;43:407–424. [PubMed] [Google Scholar]
- Taminiau J. A., Gall D. G., Hamilton J. R. Response of the rat small-intestine epithelium to methotrexate. Gut. 1980 Jun;21(6):486–492. doi: 10.1136/gut.21.6.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson A. J. Necrosis and apoptosis in the gastrointestinal tract. Gut. 1995 Aug;37(2):165–167. doi: 10.1136/gut.37.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]


