Abstract
BACKGROUND—Alone among autonomic neurones, enteric neurones are known to be vulnerable to age related cell death; over 50% may be lost in aging rodents. A previous study demonstrated unexpectedly that neurones of the myenteric plexus from rats fed a restricted diet appeared not to suffer from extensive cell death in contrast with previous studies of ad libitum fed animals. AIMS—To compare myenteric neurone numbers in the ileum of young and aging male Sprague-Dawley rats fed either ad libitum or a restricted diet. METHODS—Neurones were counted in whole mount preparations of rat ileum stained immunohistochemically for the pan-neuronal marker PGP9.5, for choline acetyltransferase, or for nitric oxide synthase, or with NADH or NADPH histochemistry. RESULTS—Neurone numbers in the rat myenteric plexus were substantially affected by the dietary regimen: ad libitum feeding (50-60 g per day of standard rat chow) resulted in the death of about 50% of myenteric neurones in 24 month Sprague-Dawley rats, while numbers were unchanged when the daily dietary intake was halved between the ages of six and 24 months. Animals fed a double restricted diet (15 g per day) showed no cell loss at 30 months, as well as the predicted increase in longevity. Neurone loss was largely complete by 16 months in ad libitum fed animals. Numbers of cholinergic (possibly motor) neurones, as demonstrated by choline acetyltransferase immunohistochemistry, were substantially reduced in ad libitum fed aging rats but not in animals fed a restricted diet. Loss of cholinergic neurones after ad libitum feeding was confirmed by reduced numbers of neurones of a size range matching that of cholinergic neurones. CONCLUSIONS—Ad libitum feeding of adult rats has adverse effects on the survival of myenteric neurones, neurone loss commencing before 16 months of age. Cholinergic neurones appear to be particularly vulnerable to the effects of diet. Restricting dietary intake from six months of age prevents neurone loss almost entirely up to 30 months of age in these rats. Keywords: restricted diet; enteric neurones; aging; neuronal cell death
Full Text
The Full Text of this article is available as a PDF (252.6 KB).
Figure 1 .
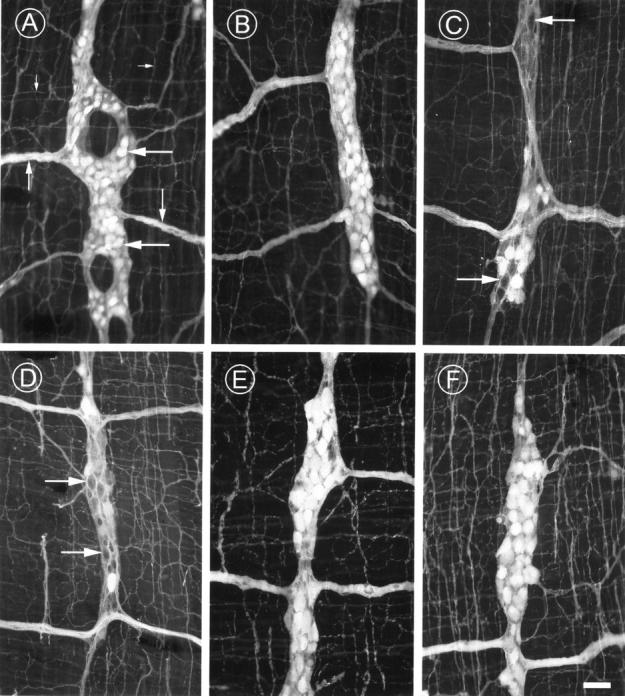
Photomicrographs showing PGP immunoreactive neurones in the myenteric plexus of young (4-6 month (A)), ad libitum (AL) fed (AL 16 month (B, C), AL 24 month (D)), and restricted diet (RD 24 month (E) and double RD 30 month (F)) rats. Note the ganglion full of brightly stained neurones in (A) (large arrows), and also in (B), (E), and (F). The secondary (medium arrows) and tertiary (small arrows) plexuses are also seen in all preparations. Nerve loss is clearly seen as spaces within the majority of ganglia in AL 16 month and AL 24 month animals (C, D) (arrows) but not in all AL animals (B). Scale bar 50 µm.
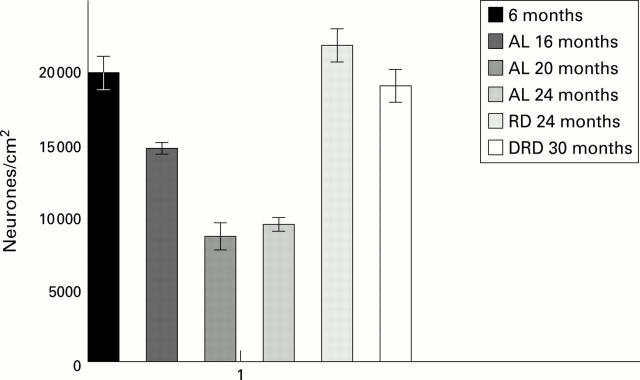
Figure 2 .
Histogram showing the number of PGP immunoreactive neurones in the myenteric plexus of young (4-6 month) rats, ad libitum fed (AL) rats at 16, 20, and 24 months, rats fed a restricted diet (RD) at 24 months, and rats fed a double restricted diet (DRD) at 30 months. Note the comparable loss of neurones in AL 16, AL 20, and AL 24 month groups compared with young, RD 24 month and DRD 30 month groups. These losses were highly significant (see table 2), reaching >50% in the AL groups. Also, note the lack of cell loss in senile (DRD 30 months) animals fed a double restricted diet.
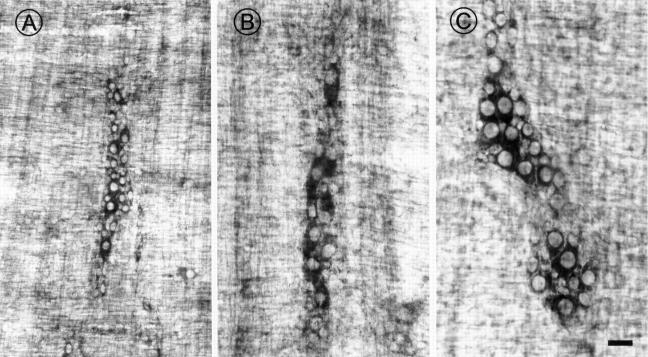
Figure 3 .
Photomicrographs showing NADH histochemical staining in the myenteric plexus of young 4-6 month (A), old ad libitum fed (AL 24 month) (B), and restricted diet (RD 24 month) (C) rats. Note the reduced density of neurones in ganglia from AL (B) as well as RD (C) rats, indicating that NADH is not suitable as a pan-neuronal marker (see text and table 1). Also, note the increased size of neurones in (B) and (C). Scale bar 50 µm.
Figure 4 .
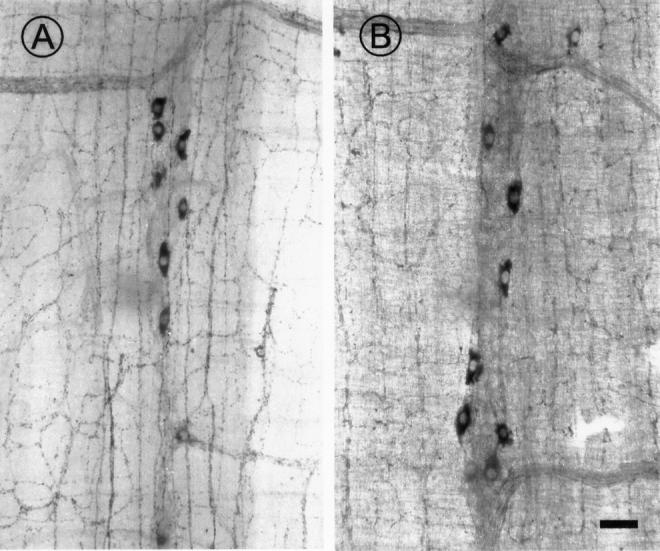
Photomicrographs showing NADPH histochemical staining for nitrergic neurones in ganglia from the myenteric plexus of young (A) and ad libitum fed 24 month (B) rats. Note the comparable density of stained neurones. Scale bar 50 µm.
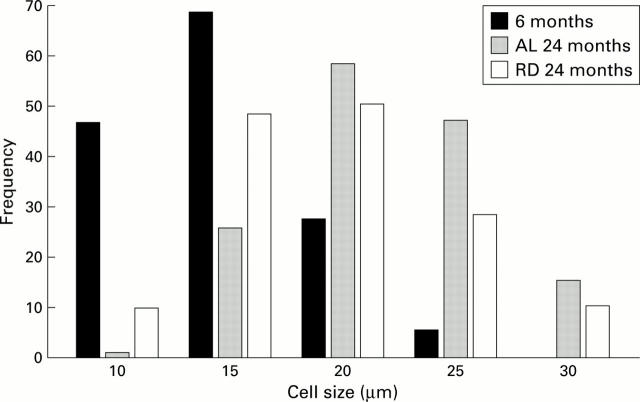
Figure 5 .
Histogram showing size (average diameter) distributions of neurones in different age groups following ad libitum (AL) and restricted diet (RD) feeding in 24 month old rats. Note the rightward shift of neurone sizes at all ages after six months, indicating continued neurone growth in adult life. The frequency of neurones of 15-17.5 µm diameter is reduced in the 24 month AL group compared with the 24 month RD group, matching the average size of cholinergic neurones measured separately in neurones from 24 month RD rats (data not shown).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aimi Y., Kimura H., Kinoshita T., Minami Y., Fujimura M., Vincent S. R. Histochemical localization of nitric oxide synthase in rat enteric nervous system. Neuroscience. 1993 Mar;53(2):553–560. doi: 10.1016/0306-4522(93)90220-a. [DOI] [PubMed] [Google Scholar]
- Baker D. M., Watson S. P., Santer R. M. Evidence for a decrease in sympathetic control of intestinal function in the aged rat. Neurobiol Aging. 1991 Jul-Aug;12(4):363–365. doi: 10.1016/0197-4580(91)90023-d. [DOI] [PubMed] [Google Scholar]
- Balaskas C., Saffrey M. J., Burnstock G. Distribution and colocalization of NADPH-diaphorase activity, nitric oxide synthase immunoreactivity, and VIP immunoreactivity in the newly hatched chicken gut. Anat Rec. 1995 Sep;243(1):10–18. doi: 10.1002/ar.1092430103. [DOI] [PubMed] [Google Scholar]
- Barnard R. J., Youngren J. F., Martin D. A. Diet, not aging, causes skeletal muscle insulin resistance. Gerontology. 1995;41(4):205–211. doi: 10.1159/000213683. [DOI] [PubMed] [Google Scholar]
- Belai A., Cooper S., Burnstock G. Effect of age on NADPH-diaphorase-containing myenteric neurones of rat ileum and proximal colon. Cell Tissue Res. 1995 Feb;279(2):379–383. doi: 10.1007/BF00318495. [DOI] [PubMed] [Google Scholar]
- Brookes S. J. Neuronal nitric oxide in the gut. J Gastroenterol Hepatol. 1993 Nov-Dec;8(6):590–603. doi: 10.1111/j.1440-1746.1993.tb01658.x. [DOI] [PubMed] [Google Scholar]
- Böckelmann R., Wolf G., Ransmayr G., Riederer P. NADPH-diaphorase/nitric oxide synthase containing neurons in normal and Parkinson's disease putamen. J Neural Transm Park Dis Dement Sect. 1994;7(2):115–121. doi: 10.1007/BF02260966. [DOI] [PubMed] [Google Scholar]
- Cowen T., Haven A. J., Burnstock G. Pontamine sky blue: a counterstain for background autofluorescence in fluorescence and immunofluorescence histochemistry. Histochemistry. 1985;82(3):205–208. doi: 10.1007/BF00501396. [DOI] [PubMed] [Google Scholar]
- Cracco C., Filogamo G. Quantitative study of the NADPH-diaphorase-positive myenteric neurons of the rat ileum. Neuroscience. 1994 Jul;61(2):351–359. doi: 10.1016/0306-4522(94)90236-4. [DOI] [PubMed] [Google Scholar]
- Dalkara T., Moskowitz M. A. Neurotoxic and neuroprotective roles of nitric oxide in cerebral ischaemia. Int Rev Neurobiol. 1997;40:319–336. doi: 10.1016/s0074-7742(08)60726-6. [DOI] [PubMed] [Google Scholar]
- Eaker E. Y., Sallustio J. E. The distribution of novel intermediate filament proteins defines subpopulations of myenteric neurons in rat intestine. Gastroenterology. 1994 Sep;107(3):666–674. doi: 10.1016/0016-5085(94)90113-9. [DOI] [PubMed] [Google Scholar]
- Enesco H. E., Kruk P. Dietary restriction reduces fluorescent age pigment accumulation in mice. Exp Gerontol. 1981;16(4):357–361. doi: 10.1016/0531-5565(81)90056-5. [DOI] [PubMed] [Google Scholar]
- Fehér E., Pénzes L. Density of substance P, vasoactive intestinal polypeptide and somatostatin-containing nerve fibers in the ageing small intestine of the rats. Gerontology. 1987;33(6):341–348. doi: 10.1159/000212901. [DOI] [PubMed] [Google Scholar]
- Furness J. B., Li Z. S., Young H. M., Förstermann U. Nitric oxide synthase in the enteric nervous system of the guinea-pig: a quantitative description. Cell Tissue Res. 1994 Jul;277(1):139–149. doi: 10.1007/BF00303090. [DOI] [PubMed] [Google Scholar]
- Gabella G. Detection of nerve cells by a histochemical technic. Experientia. 1969 Feb 15;25(2):218–219. doi: 10.1007/BF01899135. [DOI] [PubMed] [Google Scholar]
- Gabella G. Fall in the number of myenteric neurons in aging guinea pigs. Gastroenterology. 1989 Jun;96(6):1487–1493. doi: 10.1016/0016-5085(89)90516-7. [DOI] [PubMed] [Google Scholar]
- Gabella G. Neuron size and number in the myenteric plexus of the newborn and adult rat. J Anat. 1971 May;109(Pt 1):81–95. [PMC free article] [PubMed] [Google Scholar]
- Gomes O. A., de Souza R. R., Liberti E. A. A preliminary investigation of the effects of aging on the nerve cell number in the myenteric ganglia of the human colon. Gerontology. 1997;43(4):210–217. doi: 10.1159/000213852. [DOI] [PubMed] [Google Scholar]
- Horowitz M., Maddern G. J., Chatterton B. E., Collins P. J., Harding P. E., Shearman D. J. Changes in gastric emptying rates with age. Clin Sci (Lond) 1984 Aug;67(2):213–218. doi: 10.1042/cs0670213. [DOI] [PubMed] [Google Scholar]
- Hosoda S., Bamba T., Nakago S., Fujiyma Y., Senda S., Hirata M. Age-related changes in the gastrointestinal tract. Nutr Rev. 1992 Dec;50(12):374–377. doi: 10.1111/j.1753-4887.1992.tb02484.x. [DOI] [PubMed] [Google Scholar]
- Iadecola C. Bright and dark sides of nitric oxide in ischemic brain injury. Trends Neurosci. 1997 Mar;20(3):132–139. doi: 10.1016/s0166-2236(96)10074-6. [DOI] [PubMed] [Google Scholar]
- Johnson R. J., Schemann M., Santer R. M., Cowen T. The effects of age on the overall population and on sub-populations of myenteric neurons in the rat small intestine. J Anat. 1998 May;192(Pt 4):479–488. doi: 10.1046/j.1469-7580.1998.19240479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaosmanoglu T., Aygun B., Wade P. R., Gershon M. D. Regional differences in the number of neurons in the myenteric plexus of the guinea pig small intestine and colon: an evaluation of markers used to count neurons. Anat Rec. 1996 Apr;244(4):470–480. doi: 10.1002/(SICI)1097-0185(199604)244:4<470::AID-AR5>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Lehéricy S., Hirsch E. C., Cervera P., Hersh L. B., Hauw J. J., Ruberg M., Agid Y. Selective loss of cholinergic neurons in the ventral striatum of patients with Alzheimer disease. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8580–8584. doi: 10.1073/pnas.86.21.8580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen J. L. Effects of gender, age, and body mass index on gastrointestinal transit times. Dig Dis Sci. 1992 Oct;37(10):1548–1553. doi: 10.1007/BF01296501. [DOI] [PubMed] [Google Scholar]
- McDougal J. N., Miller M. S., Burks T. F., Kreulen D. L. Age-related changes in colonic function in rats. Am J Physiol. 1984 Nov;247(5 Pt 1):G542–G546. doi: 10.1152/ajpgi.1984.247.5.G542. [DOI] [PubMed] [Google Scholar]
- Meciano Filho J., Carvalho V. C., de Souza R. R. Nerve cell loss in the myenteric plexus of the human esophagus in relation to age: a preliminary investigation. Gerontology. 1995;41(1):18–21. doi: 10.1159/000213658. [DOI] [PubMed] [Google Scholar]
- Merkel I. S., Locher J., Burgio K., Towers A., Wald A. Physiologic and psychologic characteristics of an elderly population with chronic constipation. Am J Gastroenterol. 1993 Nov;88(11):1854–1859. [PubMed] [Google Scholar]
- Nowak T. V., Harrington B., Kalbfleisch J. Age-related changes in enteric neuromuscular transmission. J Pharmacol Exp Ther. 1990 May;253(2):683–687. [PubMed] [Google Scholar]
- Pichel J. G., Shen L., Sheng H. Z., Granholm A. C., Drago J., Grinberg A., Lee E. J., Huang S. P., Saarma M., Hoffer B. J. Defects in enteric innervation and kidney development in mice lacking GDNF. Nature. 1996 Jul 4;382(6586):73–76. doi: 10.1038/382073a0. [DOI] [PubMed] [Google Scholar]
- Porter A. J., Wattchow D. A., Brookes S. J., Schemann M., Costa M. Choline acetyltransferase immunoreactivity in the human small and large intestine. Gastroenterology. 1996 Aug;111(2):401–408. doi: 10.1053/gast.1996.v111.pm8690205. [DOI] [PubMed] [Google Scholar]
- Roth G. S., Ingram D. K., Joseph J. A. Delayed loss of striatal dopamine receptors during aging of dietarily restricted rats. Brain Res. 1984 May 21;300(1):27–32. doi: 10.1016/0006-8993(84)91337-4. [DOI] [PubMed] [Google Scholar]
- Santer R. M., Baker D. M. Enteric neuron numbers and sizes in Auerbach's plexus in the small and large intestine of adult and aged rats. J Auton Nerv Syst. 1988 Nov;25(1):59–67. doi: 10.1016/0165-1838(88)90008-2. [DOI] [PubMed] [Google Scholar]
- Santer R. M., Conboy V. B. Prenatal undernutrition permanently decreases enteric neuron number and sympathetic innervation of Auerbach's plexus in the rat. J Anat. 1990 Feb;168:57–62. [PMC free article] [PubMed] [Google Scholar]
- Santer R. M. Survival of the population of NADPH-diaphorase stained myenteric neurons in the small intestine of aged rats. J Auton Nerv Syst. 1994 Oct;49(2):115–121. doi: 10.1016/0165-1838(94)90131-7. [DOI] [PubMed] [Google Scholar]
- Schemann M., Sann H., Schaaf C., Mäder M. Identification of cholinergic neurons in enteric nervous system by antibodies against choline acetyltransferase. Am J Physiol. 1993 Nov;265(5 Pt 1):G1005–G1009. doi: 10.1152/ajpgi.1993.265.5.G1005. [DOI] [PubMed] [Google Scholar]
- Siou G. P., Belai A., Burnstock G. Age-related changes in galanin- and calretinin-immunoreactive nerves of guinea-pig gallbladder. Neuroreport. 1992 Nov;3(11):990–992. doi: 10.1097/00001756-199211000-00011. [DOI] [PubMed] [Google Scholar]
- Steele P. A., Brookes S. J., Costa M. Immunohistochemical identification of cholinergic neurons in the myenteric plexus of guinea-pig small intestine. Neuroscience. 1991;45(1):227–239. doi: 10.1016/0306-4522(91)90119-9. [DOI] [PubMed] [Google Scholar]
- Stokkan K. A., Reiter R. J., Nonaka K. O., Lerchl A., Yu B. P., Vaughan M. K. Food restriction retards aging of the pineal gland. Brain Res. 1991 Apr 5;545(1-2):66–72. doi: 10.1016/0006-8993(91)91270-b. [DOI] [PubMed] [Google Scholar]
- Warren M. A. Adaptations of the rat small intestine to a single and a double period of undernutrition. J Anat. 1991 Jun;176:89–97. [PMC free article] [PubMed] [Google Scholar]
- Warren M. A., Bedi K. S. Synapse-to-neuron ratios in the visual cortex of adult rats undernourished from about birth until 100 days of age. J Comp Neurol. 1982 Sep 1;210(1):59–64. doi: 10.1002/cne.902100107. [DOI] [PubMed] [Google Scholar]
- Weindruch R. The retardation of aging by caloric restriction: studies in rodents and primates. Toxicol Pathol. 1996 Nov-Dec;24(6):742–745. doi: 10.1177/019262339602400618. [DOI] [PubMed] [Google Scholar]
- de Souza R. R., Moratelli H. B., Borges N., Liberti E. A. Age-induced nerve cell loss in the myenteric plexus of the small intestine in man. Gerontology. 1993;39(4):183–188. doi: 10.1159/000213532. [DOI] [PubMed] [Google Scholar]