Abstract
INTRODUCTION—Epidermal growth factor (EGF) is normally present as EGF1-53. A variety of C terminal truncated forms have been used in preliminary trials for treating gastrointestinal injury but their relative potency and stability when used in a clinical setting are unclear. Therefore, we compared the biological activity of recombinant EGF1-53, EGF1-52, EGF1-51, and the C terminal peptides EGF44-53 and EGF49-53. METHODS—Purity of forms was confirmed by mass spectrometry. Bioactivity of the different EGF forms was determined using [methyl-3H] thymidine incorporation into primary rat hepatocytes and their ability to reduce indomethacin (20 mg/kg subcutaneously)/restraint induced gastric injury in rats. Stability of EGF peptides was determined by serial sampling from a syringe driver system containing EGF/4% albumin in saline. RESULTS—Biological activity assays of EGF1-53, EGF1-52, and EGF1-51 gave almost identical thymidine uptake dose-response curves (maximal responses increasing baseline uptake from 4400 (600) cpm (mean (SEM)) to about 22 000 (2000) cpm when EGF was added at 1.6 nM). EGF44-53 and EGF49-53 did not stimulate 3H thymidine uptake. Control rats had 47 (4) mm2 damage/stomach, EGF1-51, EGF1-52, and EGF1-53 at 0.16 and 0.80 nmol/kg/h each reduced gastric injury by about 50% and 80%, respectively (both doses p<0.01 compared with control but no significant difference between the different forms). EGF was stable at room temperature for seven days but biological activity decreased by 35% and 40% at two and three weeks, respectively (both p<0.01). Exposure to light did not affect bioactivity. CONCLUSION—EGF1-51 and EGF1-52 are as biologically active as full length EGF1-53 but the C terminal penta- and decapeptides are ineffective. Clinical trials of EGF can probably use infusion systems for at least 48 hours at room temperature and with exposure to light, without reducing biological efficacy. Keywords: epidermal growth factor; intestinal injury; nutrition
Full Text
The Full Text of this article is available as a PDF (150.3 KB).
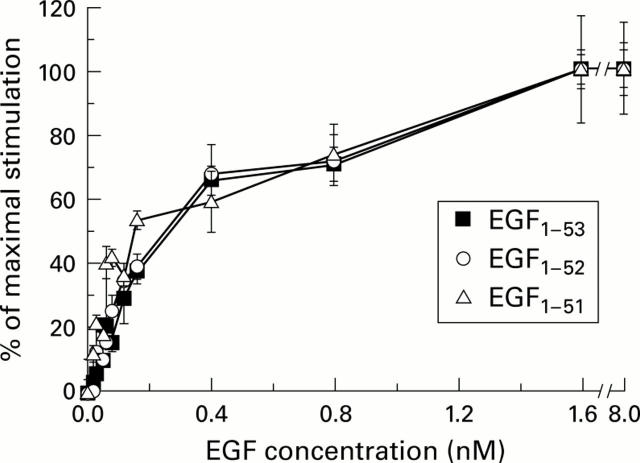
Figure 1 .
Effect of epidermal growth factors EGF1-53, EGF1-52, and EGF1-51 on [methyl-3H] thymidine uptake into primary rat hepatocytes. The dose-response curves of the various forms of EGF are virtually superimposable. Results are expressed as mean (SEM), n=4.
Figure 2 .
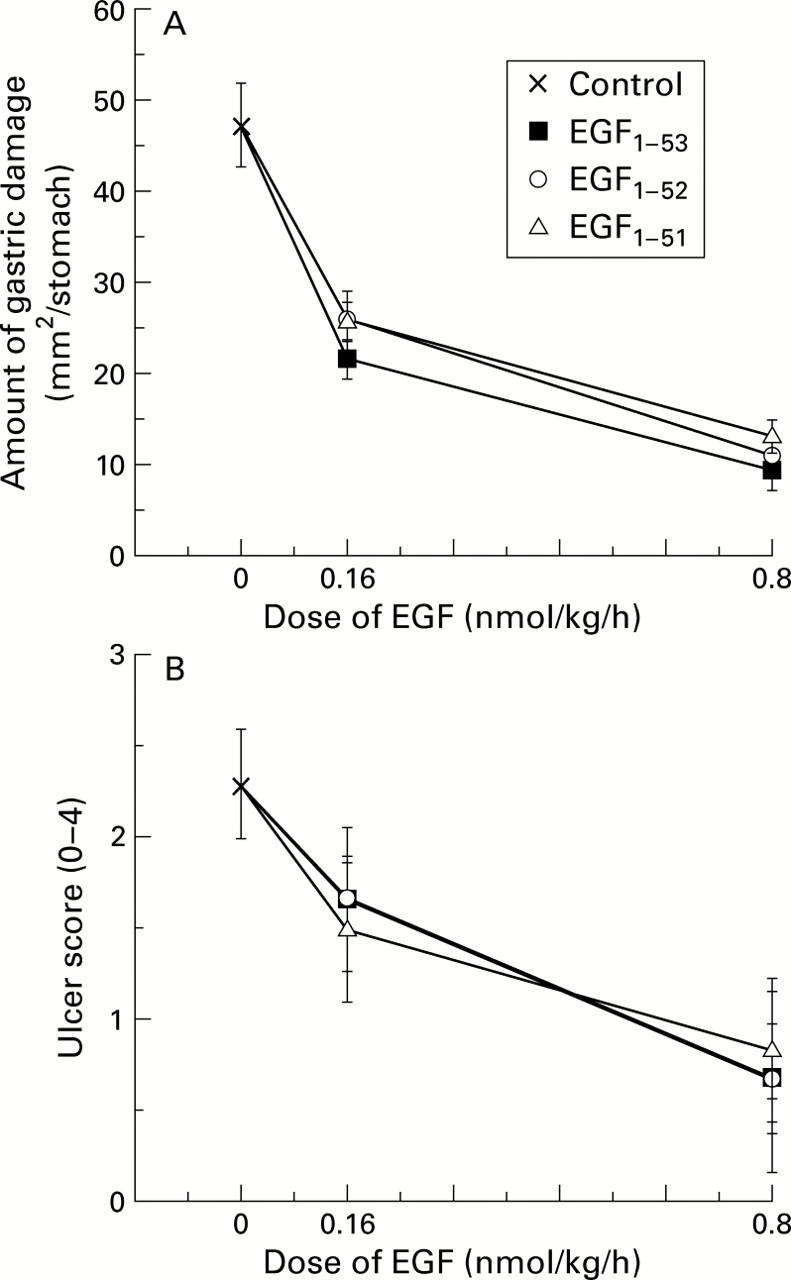
Comparison of the biological activity of various forms of epidermal growth factor (EGF) using attenuation of gastric damage as a bioassay. Rats were restrained and given a continuous subcutaneous infusion of saline (control), EGF1-53, EGF1-52, or EGF1-51. Thirty minutes later, all animals also received indomethacin (20 mg/kg subcutaneously). Three hours after administration of indomethacin, all animals were killed and the amount of gastric injury assessed. (A) All forms of EGF reduced the amount of total ulcerated area (mm2/stomach) (all p<0.01 v control) but there were no differences in potency between the forms. (B) Assessment using a microscopic damage score, where each stomach was given a score of 0-4 (0, no damage; 1, one small erosion (less than 0.5 mm); 2, two small or one large erosion (greater than 0.5 mm); 3, two or more large erosions; and 4, any area of ulceration extending to the muscularis mucosa). Results are expressed as mean (SEM), n=6 animals per datum point.
Figure 3 .
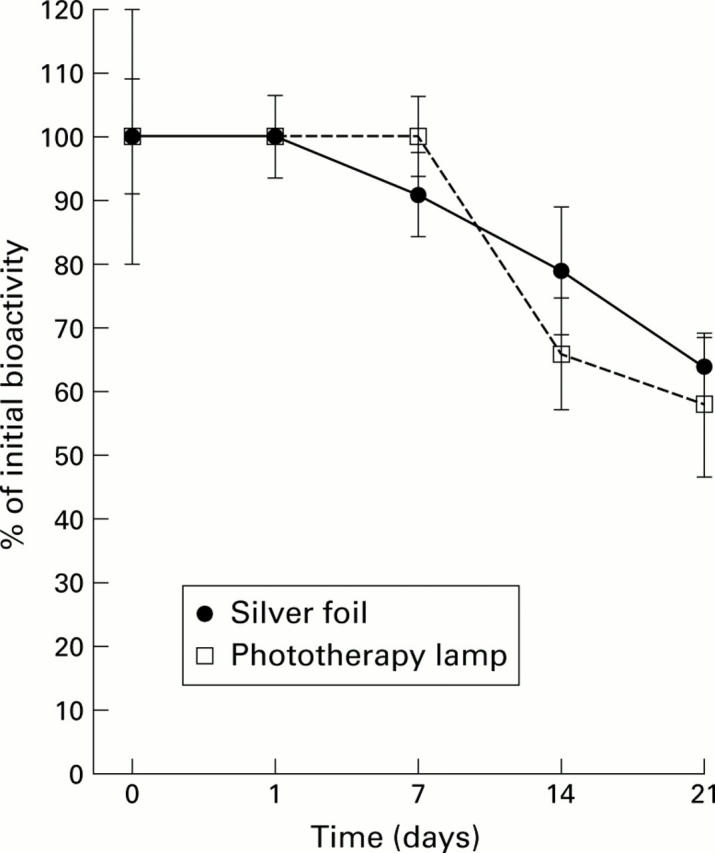
Stability of epidermal growth factor (EGF) when placed in a clinical setting. A 60:40 mixture of EGF1-51 and EGF1-52 is commercially available in an intravenous formulation. To determine its stability under likely clinical conditions, the EGF1-51/EGF1-52 mixture was diluted to 1 µg/ml in a 4.5% solution of human albumin and divided into two samples. The test solutions were pumped at room temperature (24-26°C) through an infusion system. One infusion system was covered in silver foil and the other was placed under a phototherapy lamp to mimic the conditions of phototherapy treatment commonly prescribed for the jaundiced neonate. Samples were subsequently analysed for biological activity using the rat hepatocyte assay. Results are expressed as mean (SEM), n=4.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Araki F., Nakamura H., Nojima N., Tsukumo K., Sakamoto S. Stability of recombinant human epidermal growth factor in various solutions. Chem Pharm Bull (Tokyo) 1989 Feb;37(2):404–406. doi: 10.1248/cpb.37.404. [DOI] [PubMed] [Google Scholar]
- Bell G. I., Fong N. M., Stempien M. M., Wormsted M. A., Caput D., Ku L. L., Urdea M. S., Rall L. B., Sanchez-Pescador R. Human epidermal growth factor precursor: cDNA sequence, expression in vitro and gene organization. Nucleic Acids Res. 1986 Nov 11;14(21):8427–8446. doi: 10.1093/nar/14.21.8427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drumm B., Cutz E., Tomkins K. B., Cook D., Hamilton J. R., Sherman P. Urogastrone/epidermal growth factor in treatment of congenital microvillous atrophy. Lancet. 1988 Jan 16;1(8577):111–112. doi: 10.1016/s0140-6736(88)90303-0. [DOI] [PubMed] [Google Scholar]
- Goodlad R. A., Boulton R., Playford R. J. Comparison of the mitogenic activity of human epidermal growth factor I-53 and epidermal growth factor I-48 in vitro and in vivo. Clin Sci (Lond) 1996 Oct;91(4):503–507. doi: 10.1042/cs0910503. [DOI] [PubMed] [Google Scholar]
- Gregory H., Thomas C. E., Young J. A., Willshire I. R., Garner A. The contribution of the C-terminal undecapeptide sequence of urogastrone-epidermal growth factor to its biological action. Regul Pept. 1988 Aug;22(3):217–226. doi: 10.1016/0167-0115(88)90034-1. [DOI] [PubMed] [Google Scholar]
- Haedo W., González T., Más J. A., Franco S., Gra B., Soto G., Alonso A., López-Saura P. Oral human recombinant epidermal growth factor in the treatment of patients with duodenal ulcer. Rev Esp Enferm Dig. 1996 Jun;88(6):409–418. [PubMed] [Google Scholar]
- Heitz P. U., Kasper M., van Noorden S., Polak J. M., Gregory H., Pearse A. G. Immunohistochemical localisation of urogastrone to human duodenal and submandibular glands. Gut. 1978 May;19(5):408–413. doi: 10.1136/gut.19.5.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenberg M. D., Gregory H. Epidermal growth factor-urogastrone: biological activity and receptor binding of derivatives. Mol Pharmacol. 1980 May;17(3):314–320. [PubMed] [Google Scholar]
- Itoh M., Matsuo Y. Gastric ulcer treatment with intravenous human epidermal growth factor: a double-blind controlled clinical study. J Gastroenterol Hepatol. 1994;9 (Suppl 1):S78–S83. doi: 10.1111/j.1440-1746.1994.tb01307.x. [DOI] [PubMed] [Google Scholar]
- Joh T., Itoh M., Katsumi K., Yokoyama Y., Takeuchi T., Kato T., Wada Y., Tanaka R. Physiological concentrations of human epidermal growth factor in biological fluids: use of a sensitive enzyme immunoassay. Clin Chim Acta. 1986 Jul 15;158(1):81–90. doi: 10.1016/0009-8981(86)90118-x. [DOI] [PubMed] [Google Scholar]
- Kelly S. M., Jenner J. R., Dickinson R. J., Hunter J. O. Increased gastric juice epidermal growth factor after non-steroidal anti-inflammatory drug ingestion. Gut. 1994 May;35(5):611–614. doi: 10.1136/gut.35.5.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck M. S., Bass P. Effect of epidermal growth factor on experimental colitis in the rat. J Pharmacol Exp Ther. 1993 Feb;264(2):984–990. [PubMed] [Google Scholar]
- Matsunami R. K., Yette M. L., Stevens A., Niyogi S. K. Mutational analysis of leucine 47 in human epidermal growth factor. J Cell Biochem. 1991 Jul;46(3):242–249. doi: 10.1002/jcb.240460307. [DOI] [PubMed] [Google Scholar]
- Pappin D. J., Hojrup P., Bleasby A. J. Rapid identification of proteins by peptide-mass fingerprinting. Curr Biol. 1993 Jun 1;3(6):327–332. doi: 10.1016/0960-9822(93)90195-t. [DOI] [PubMed] [Google Scholar]
- Playford R. J., Hanby A. M., Gschmeissner S., Peiffer L. P., Wright N. A., McGarrity T. The epidermal growth factor receptor (EGF-R) is present on the basolateral, but not the apical, surface of enterocytes in the human gastrointestinal tract. Gut. 1996 Aug;39(2):262–266. doi: 10.1136/gut.39.2.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Playford R. J., Marchbank T., Calnan D. P., Calam J., Royston P., Batten J. J., Hansen H. F. Epidermal growth factor is digested to smaller, less active forms in acidic gastric juice. Gastroenterology. 1995 Jan;108(1):92–101. doi: 10.1016/0016-5085(95)90012-8. [DOI] [PubMed] [Google Scholar]
- Playford R. J. Recombinant peptides for gastrointestinal ulceration: still early days. Gut. 1997 Feb;40(2):286–287. doi: 10.1136/gut.40.2.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Playford R. J., Vesey D. A., Haldane S., Alison M. R., Calam J. Dose-dependent effects of fentanyl on indomethacin-induced gastric damage. Digestion. 1991;49(4):198–203. doi: 10.1159/000200722. [DOI] [PubMed] [Google Scholar]
- Playford R. J., Woodman A. C., Clark P., Watanapa P., Vesey D., Deprez P. H., Williamson R. C., Calam J. Effect of luminal growth factor preservation on intestinal growth. Lancet. 1993 Apr 3;341(8849):843–848. doi: 10.1016/0140-6736(93)93057-8. [DOI] [PubMed] [Google Scholar]
- Richman R. A., Claus T. H., Pilkis S. J., Friedman D. L. Hormonal stimulation of DNA synthesis in primary cultures of adult rat hepatocytes. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3589–3593. doi: 10.1073/pnas.73.10.3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan P. B., Brueton M. J., Tabara Z. B., Goodlad R. A., Lee C. Y., Wright N. A. Epidermal growth factor in necrotising enteritis. Lancet. 1991 Jul 6;338(8758):53–54. doi: 10.1016/0140-6736(91)90042-n. [DOI] [PubMed] [Google Scholar]
- Tsukumo K., Nakamura H., Sakamoto S. Purification and characterization of high molecular weight human epidermal growth factor from human urine. Biochem Biophys Res Commun. 1987 May 29;145(1):126–133. doi: 10.1016/0006-291x(87)91296-4. [DOI] [PubMed] [Google Scholar]
- Walker-Smith J. A., Phillips A. D., Walford N., Gregory H., Fitzgerald J. D., MacCullagh K., Wright N. A. Intravenous epidermal growth factor/urogastrone increases small-intestinal cell proliferation in congenital microvillous atrophy. Lancet. 1985 Nov 30;2(8466):1239–1240. doi: 10.1016/s0140-6736(85)90762-7. [DOI] [PubMed] [Google Scholar]
- van Zoelen E. J., Lenferink A. E., Kramer R. H., van de Poll M. L. Rational design for the development of epidermal growth factor receptor antagonists. Pathol Res Pract. 1996 Jul;192(7):761–767. doi: 10.1016/S0344-0338(96)80098-7. [DOI] [PubMed] [Google Scholar]