Abstract
BACKGROUND—Therapeutic or accidental exposure to radiation commonly causes gastrointestinal disturbances, including diarrhoea. Rats subjected to whole body ionising radiation at a dose of 8 Gy lose their capacity to absorb fluid via the descending colon after four days. After seven days, fluid absorption recovers to control levels. AIMS—To investigate the effect of ionising radiation on colonic permeability together with its effect on mitochondria dependent apoptotic signals and intercellular adhesion molecules. METHODS—Rats were irradiated with doses of 0-12 Gy. Colonic permeability was measured by accumulation of fluorescein isothiocyanate (FITC) dextran in crypt lumens. Changes in levels of cytochrome c, caspase 3, E and OB cadherin, β-catenin smooth muscle actin, and collagen IV were assessed using immunocytochemistry with confocal microscopy. RESULTS—Cytosolic cytochrome c increased after 8 Gy (t1/2 1.4 (0.6) hours) and peaked at approximately six hours. Caspase 3 increased more slowly, particularly in crypt epithelial cells (t1/2 57 (14.5) hours). Pericryptal myofibroblasts disintegrated within 24 hours as was evident from loss of OB cadherin and smooth muscle actin. This coincided with increased crypt permeability to dextran. Intercellular adhesion between crypt luminal cells was not lost until day 4 when both β-catenin and E-cadherin were minimal. The half maximal dose-response for these effects was in the range 2-4 Gy. Recovery of colonic transport was concurrent with recovery of pericryptal smooth muscle actin and OB cadherin. The pan caspase inhibitor Z-Val-Ala-Asp.fluoromethylketone (1 mg/kg per day) had a small effect in conserving the pericryptal sheath myofibroblasts and sheath permeability but had no systemic therapeutic effects. CONCLUSIONS—These data suggest that radiation damage to the colon may be initiated by mitochondrial events. Loss of crypt fluid absorption and increased permeability coincided with decreased intercellular adhesion between crypt epithelial cells and loss of pericryptal sheath barrier function. Keywords: colon; cytochrome c; myofibroblast; pericryptal sheath; cadherin; radiation
Full Text
The Full Text of this article is available as a PDF (526.9 KB).
Figure 1 .
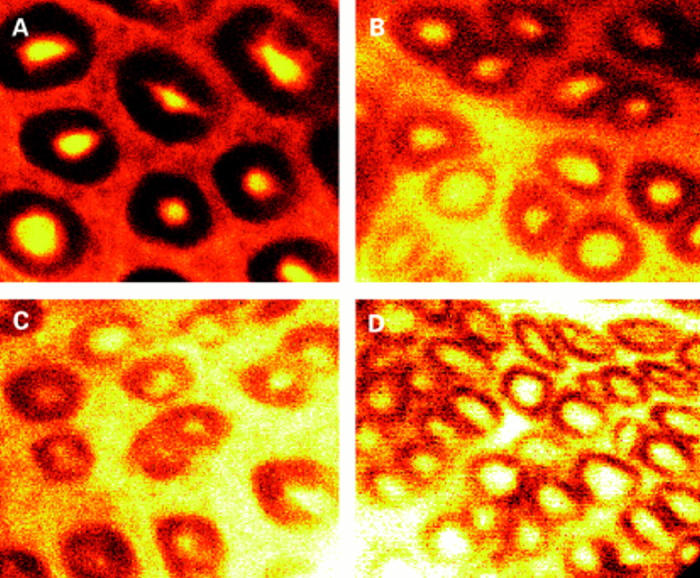
Confocal images at a depth of 40 µm from the luminal surface. (A) Control rats accumulate dextran within crypt lumens 10 times above that in external solution. Two (B) and four (C) days after radiation (8 Gy), reduced accumulation in crypt and increased leakage into the pericryptal region are seen. Seven days after irradiation (D) some recovery with increased accumulation in crypt lumens is evident.
Figure 2 .
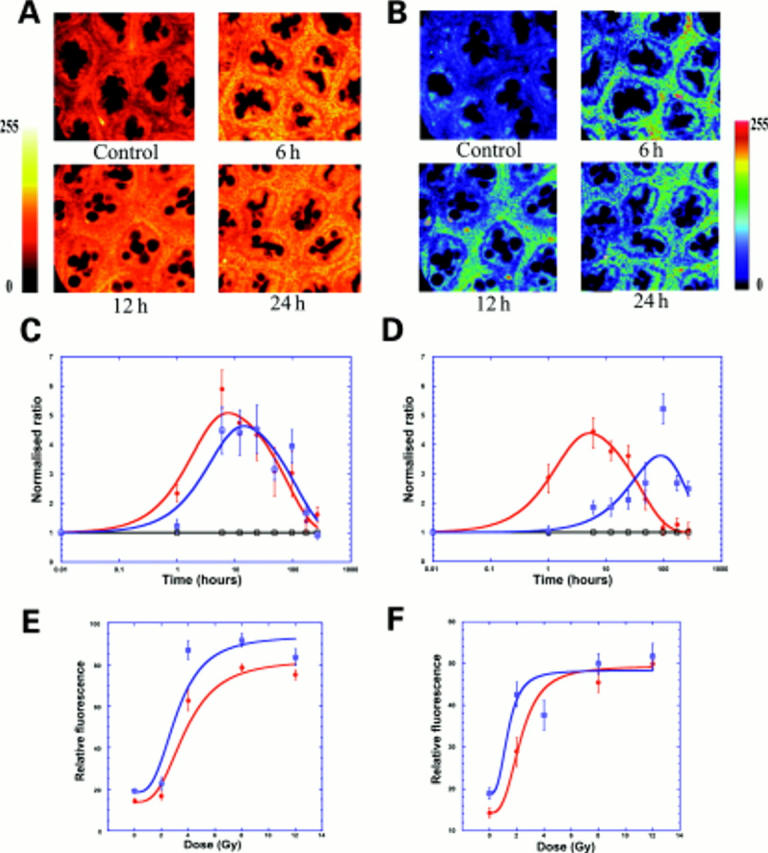
Rapid release of cytochrome c after radiation in both crypt and pericrypt regions and appearance of caspase 3. (A) Confocal images (depth 20 µm) showing cytochrome c after 8 Gy radiation in rat colon in controls and after six, 12, and 24 hours. (B) Confocal images (depth 20 µm) showing caspase 3 after 8 Gy radiation in rat colon in controls and after six, 12, and 24 hours. (C) Time course of cytochrome c (red) and caspase 3 (blue) in pericryptal sheath after 8 Gy radiation showing a small time lag between maximal increases. Data shown (n=3) as normalised ratios on a log scale against controls taken from the pericryptal region. Cytochrome c data were plotted with a biphasic exponential fit giving half times for the increase and decrease (see table 1 and materials and methods). (D) Time course of cytochrome c (red) and caspase 3 (blue) in crypt cells after 8 Gy radiation showing a large time lag between maximal increases. Data from crypt regions of same images used for (C). (E) Dose-response (0-12 Gy) curve for cytochrome c release after 12 hours in pericryptal (red) and crypt (blue) cells. Data shown as relative fluorescence (n=3) plotted with a non-linear Michaelis-Menten curve fit showing a saturable dose-response. (F) Dose-response (0-12 Gy) curve for caspase 3 after 12 hours in pericryptal (red) and crypt (blue) cells. Data shown as relative fluorescence (n=3) plotted with a non-linear Michaelis-Menten curve fit showing a saturable dose-response.
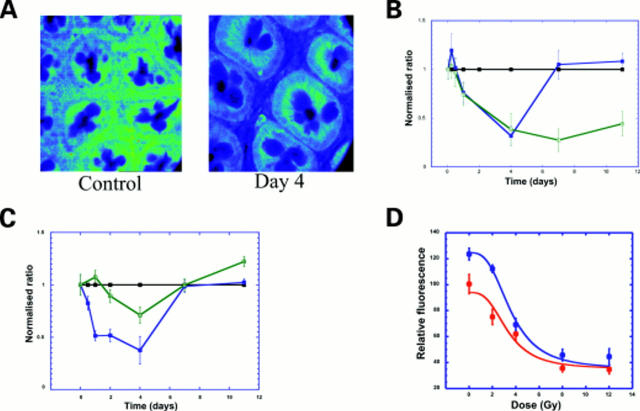
Figure 3 .
Decrease in E-cadherin and β-catenin in colon 2-4 days after radiation. (A) Confocal images (depth 20 µm) showing decrease in E-cadherin four days after radiation, particularly in the pericryptal region. (B) Time course of E-cadherin in pericryptal (blue) and crypt (green) cells after 8 Gy radiation showing a decrease in both the pericrypt and crypt and recovery in pericryptal cells. Data shown (n=3) as normalised ratios against control. (C) Time course of β-catenin in pericryptal (blue) and crypt (green) cells after 8 Gy radiation showing a decrease in both the pericrypt and crypt and recovery in both pericryptal and crypt cells. Data shown (n=3) as normalised ratios against control. (D) Dose-response of E-cadherin (0-12 Gy) showing a saturable decrease. Data shown as relative fluorescence (n=3) and plotted using a non-linear Michaelis-Menten fit.
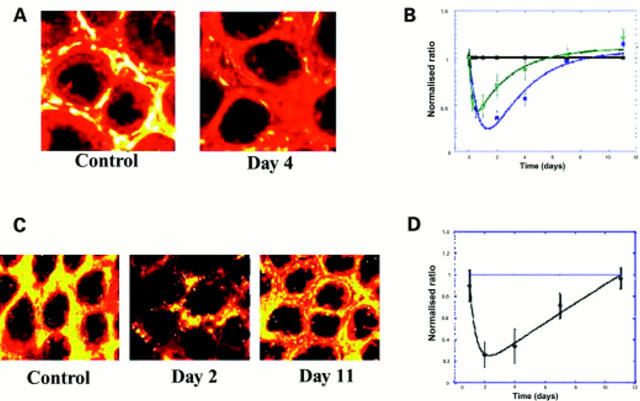
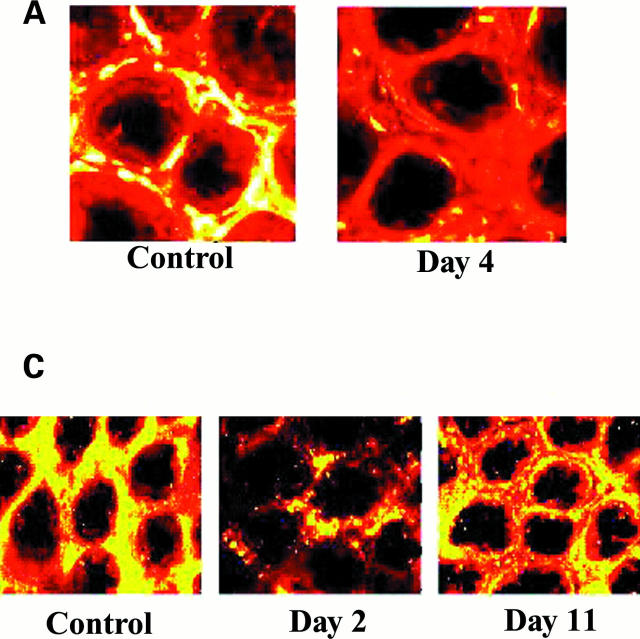
Figure 4 .
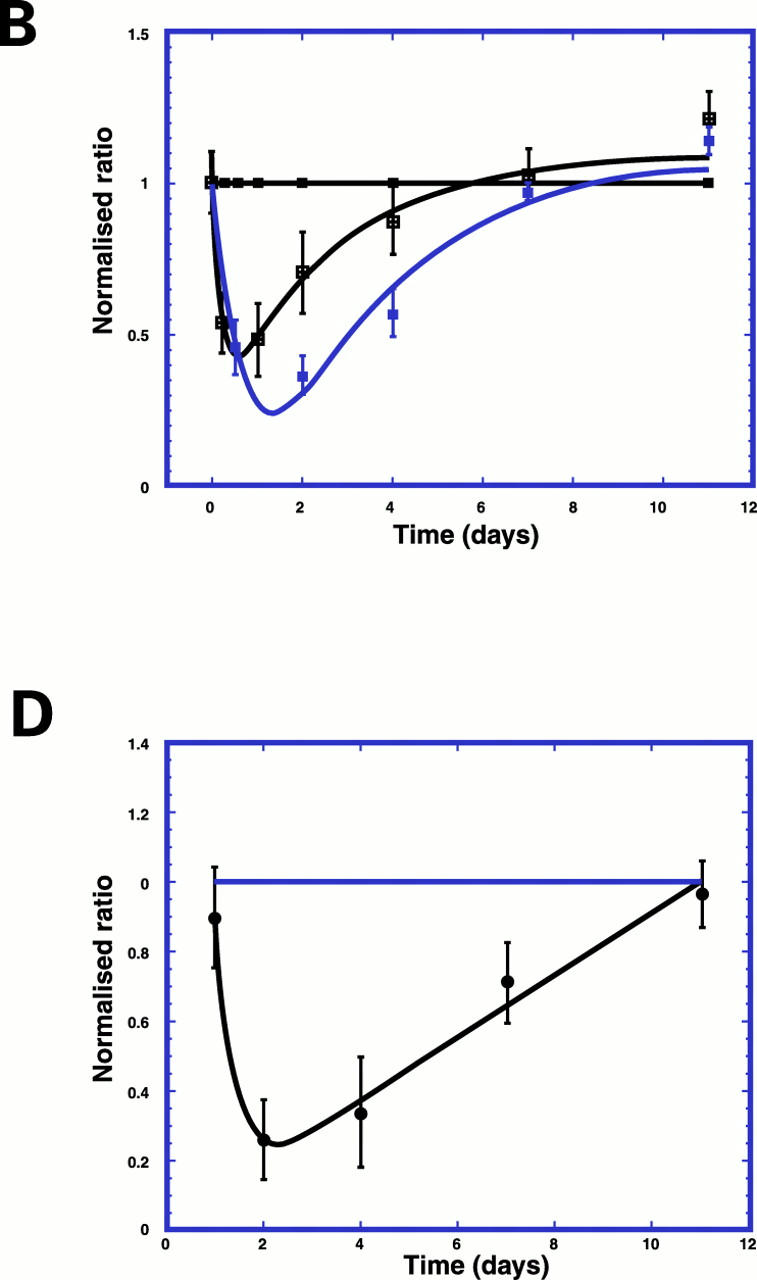
Decrease in α smooth muscle actin, OB cadherin, and collagen type IV in pericryptal sheath region after radiation. (A) Confocal images (depth 20 µm) showing decrease in smooth muscle actin in pericryptal areas after 8 Gy radiation. (B) Time course of smooth muscle actin (blue) and OB cadherin (green) in pericryptal sheath cells after 8 Gy radiation showing maximal decreases at two days and recovery after seven days. Data shown (n=3) as normalised ratios against control. (C) Confocal images (depth 20 µm) showing decrease in collagen type IV in pericryptal areas after 8 Gy radiation. (D) Time course of collagen type IV in pericryptal sheath after 8 Gy radiation showing maximal decreases at 2-4 days and recovery after 11 days. Data shown (n=3) as normalised ratios against control.
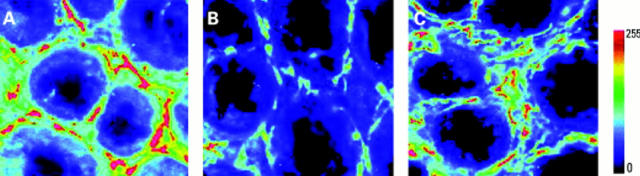
Figure 5 .
Effect of Z-vad-FMK treatment on α smooth muscle actin in the pericryptal sheath region after radiation. Confocal images (depth 20 µm) showing (A) control, (B) irradiated (8 Gy)+vehicle treated, and (C) irradiated (8 Gy)+Z-vad-FMK treated.
Figure 6 .
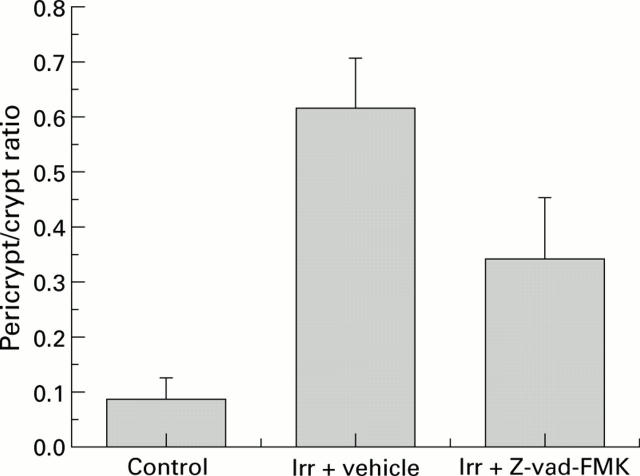
Effect of Z-vad-FMK treatment on FITC dextran accumulation after radiation. Ratio of pericrypt to crypt fluorescence in control, irradiated (irr) (8 Gy)+vehicle treated, and irradiated (8 Gy)+Z-vad-FMK treated.
Figure 7 .

Summary diagram showing possible pathway after radiation in the colon and relationship to functional changes.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boesen-de Cock J. G., Tepper A. D., de Vries E., van Blitterswijk W. J., Borst J. Common regulation of apoptosis signaling induced by CD95 and the DNA-damaging stimuli etoposide and gamma-radiation downstream from caspase-8 activation. J Biol Chem. 1999 May 14;274(20):14255–14261. doi: 10.1074/jbc.274.20.14255. [DOI] [PubMed] [Google Scholar]
- Brancolini C., Lazarevic D., Rodriguez J., Schneider C. Dismantling cell-cell contacts during apoptosis is coupled to a caspase-dependent proteolytic cleavage of beta-catenin. J Cell Biol. 1997 Nov 3;139(3):759–771. doi: 10.1083/jcb.139.3.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell S. E., Katwa L. C. Angiotensin II stimulated expression of transforming growth factor-beta1 in cardiac fibroblasts and myofibroblasts. J Mol Cell Cardiol. 1997 Jul;29(7):1947–1958. doi: 10.1006/jmcc.1997.0435. [DOI] [PubMed] [Google Scholar]
- Chaloupka R., Petit P. X., Israël N., Sureau F. Over-expression of Bcl-2 does not protect cells from hypericin photo-induced mitochondrial membrane depolarization, but delays subsequent events in the apoptotic pathway. FEBS Lett. 1999 Dec 3;462(3):295–301. doi: 10.1016/s0014-5793(99)01538-0. [DOI] [PubMed] [Google Scholar]
- D'Amours D., Germain M., Orth K., Dixit V. M., Poirier G. G. Proteolysis of poly(ADP-ribose) polymerase by caspase 3: kinetics of cleavage of mono(ADP-ribosyl)ated and DNA-bound substrates. Radiat Res. 1998 Jul;150(1):3–10. [PubMed] [Google Scholar]
- Desaki J., Fujiwara T., Komuro T. A cellular reticulum of fibroblast-like cells in the rat intestine: scanning and transmission electron microscopy. Arch Histol Jpn. 1984 Jun;47(2):179–186. doi: 10.1679/aohc.47.179. [DOI] [PubMed] [Google Scholar]
- Dublineau I., Ksas B., Aigueperse J., Gourmelon P., Griffiths N. M. In vivo alterations of fluid and electrolyte fluxes in rat colon by gamma irradiation. Dig Dis Sci. 1998 Mar;43(3):652–662. doi: 10.1023/a:1018839930552. [DOI] [PubMed] [Google Scholar]
- François A., Dublineau I., Lebrun F., Ksas B., Griffiths N. M. Modified absorptive and secretory processes in the rat distal colon after neutron irradiation: in vivo and in vitro studies. Radiat Res. 1999 Apr;151(4):468–478. [PubMed] [Google Scholar]
- Goldstein J. C., Waterhouse N. J., Juin P., Evan G. I., Green D. R. The coordinate release of cytochrome c during apoptosis is rapid, complete and kinetically invariant. Nat Cell Biol. 2000 Mar;2(3):156–162. doi: 10.1038/35004029. [DOI] [PubMed] [Google Scholar]
- Jawhari A., Farthing M., Pignatelli M. The importance of the E-cadherin-catenin complex in the maintenance of intestinal epithelial homoeostasis: more than intercellular glue? Gut. 1997 Nov;41(5):581–584. doi: 10.1136/gut.41.5.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluck R. M., Bossy-Wetzel E., Green D. R., Newmeyer D. D. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science. 1997 Feb 21;275(5303):1132–1136. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- Li P., Nijhawan D., Budihardjo I., Srinivasula S. M., Ahmad M., Alnemri E. S., Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997 Nov 14;91(4):479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- Mahida Y. R., Beltinger J., Makh S., Göke M., Gray T., Podolsky D. K., Hawkey C. J. Adult human colonic subepithelial myofibroblasts express extracellular matrix proteins and cyclooxygenase-1 and -2. Am J Physiol. 1997 Dec;273(6 Pt 1):G1341–G1348. doi: 10.1152/ajpgi.1997.273.6.G1341. [DOI] [PubMed] [Google Scholar]
- Merritt A. J., Potten C. S., Watson A. J., Loh D. Y., Nakayama K., Nakayama K., Hickman J. A. Differential expression of bcl-2 in intestinal epithelia. Correlation with attenuation of apoptosis in colonic crypts and the incidence of colonic neoplasia. J Cell Sci. 1995 Jun;108(Pt 6):2261–2271. doi: 10.1242/jcs.108.6.2261. [DOI] [PubMed] [Google Scholar]
- Munro S. B., Turner I. M., Farookhi R., Blaschuk O. W., Jothy S. E-cadherin and OB-cadherin mRNA levels in normal human colon and colon carcinoma. Exp Mol Pathol. 1995 Apr;62(2):118–122. doi: 10.1006/exmp.1995.1013. [DOI] [PubMed] [Google Scholar]
- Naftalin R. J., Pedley K. C. Regional crypt function in rat large intestine in relation to fluid absorption and growth of the pericryptal sheath. J Physiol. 1999 Jan 1;514(Pt 1):211–227. doi: 10.1111/j.1469-7793.1999.211af.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naftalin R. J., Zammit P. S., Pedley K. C. Concentration polarization of fluorescent dyes in rat descending colonic crypts: evidence of crypt fluid absorption. J Physiol. 1995 Sep 1;487(Pt 2):479–495. doi: 10.1113/jphysiol.1995.sp020894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naftalin R. J., Zammit P. S., Pedley K. C. Regional differences in rat large intestinal crypt function in relation to dehydrating capacity in vivo. J Physiol. 1999 Jan 1;514(Pt 1):201–210. doi: 10.1111/j.1469-7793.1999.201af.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascal R. R., Kaye G. I., Lane N. Colonic pericryptal fibroblast sheath: replication, migration, and cytodifferentiation of a mesenchymal cell system in adult tissue. I. Autoradiographic studies of normal rabbit colon. Gastroenterology. 1968 May;54(5):835–851. [PubMed] [Google Scholar]
- Pedley K. C., Naftalin R. J. Evidence from fluorescence microscopy and comparative studies that rat, ovine and bovine colonic crypts are absorptive. J Physiol. 1993 Jan;460:525–547. doi: 10.1113/jphysiol.1993.sp019485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piguet P. F., Vesin C., Donati Y., Barazzone C. TNF-induced enterocyte apoptosis and detachment in mice: induction of caspases and prevention by a caspase inhibitor, ZVAD-fmk. Lab Invest. 1999 Apr;79(4):495–500. [PubMed] [Google Scholar]
- Rossé T., Olivier R., Monney L., Rager M., Conus S., Fellay I., Jansen B., Borner C. Bcl-2 prolongs cell survival after Bax-induced release of cytochrome c. Nature. 1998 Jan 29;391(6666):496–499. doi: 10.1038/35160. [DOI] [PubMed] [Google Scholar]
- Sappino A. P., Schürch W., Gabbiani G. Differentiation repertoire of fibroblastic cells: expression of cytoskeletal proteins as marker of phenotypic modulations. Lab Invest. 1990 Aug;63(2):144–161. [PubMed] [Google Scholar]
- Tepper A. D., de Vries E., van Blitterswijk W. J., Borst J. Ordering of ceramide formation, caspase activation, and mitochondrial changes during CD95- and DNA damage-induced apoptosis. J Clin Invest. 1999 Apr;103(7):971–978. doi: 10.1172/JCI5457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Craen M., Declercq W., Van den brande I., Fiers W., Vandenabeele P. The proteolytic procaspase activation network: an in vitro analysis. Cell Death Differ. 1999 Nov;6(11):1117–1124. doi: 10.1038/sj.cdd.4400589. [DOI] [PubMed] [Google Scholar]
- WILSON S. G., Jr Radiation-induced gastrointestinal death in the monkey. Am J Pathol. 1959 Nov-Dec;35:1233–1251. [PMC free article] [PubMed] [Google Scholar]
- Wheelock M. J., Knudsen K. A. Cadherins and associated proteins. In Vivo. 1991 Sep-Oct;5(5):505–513. [PubMed] [Google Scholar]
- Wiernick G., Perrins D. The radiosensitivity of a mesenchymal tissue. The pericryptal fibroblast sheath in the human rectal mucosa. Br J Radiol. 1975 May;48(569):382–389. doi: 10.1259/0007-1285-48-569-382. [DOI] [PubMed] [Google Scholar]
- Yaoita H., Ogawa K., Maehara K., Maruyama Y. Attenuation of ischemia/reperfusion injury in rats by a caspase inhibitor. Circulation. 1998 Jan 27;97(3):276–281. doi: 10.1161/01.cir.97.3.276. [DOI] [PubMed] [Google Scholar]
- Zammit P. S., Mendizabal M., Naftalin R. J. Effects on fluid and Na+ flux of varying luminal hydraulic resistance in rat colon in vivo. J Physiol. 1994 Jun 15;477(Pt 3):539–548. doi: 10.1113/jphysiol.1994.sp020214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q. L., Kondo T., Noda A., Fujiwara Y. Mitochondrial and intracellular free-calcium regulation of radiation-induced apoptosis in human leukemic cells. Int J Radiat Biol. 1999 Apr;75(4):493–504. doi: 10.1080/095530099140429. [DOI] [PubMed] [Google Scholar]
- Zou H., Henzel W. J., Liu X., Lutschg A., Wang X. Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c-dependent activation of caspase-3. Cell. 1997 Aug 8;90(3):405–413. doi: 10.1016/s0092-8674(00)80501-2. [DOI] [PubMed] [Google Scholar]