Abstract
BACKGROUND AND AIMS—Constitutive cyclooxygenase (COX) 1 is believed to mediate prostaglandin dependent gastric protection. However, gastric mucosa contains cells capable of expressing inducible COX-2. We therefore investigated COX-1 and COX-2 expression, localisation, and activity in normal and abnormal human gastric mucosa. METHODS—COX-1 and COX-2 distribution was investigated by light and electron microscopic immunohistochemistry and by western blot analysis, and their contribution to prostaglandin (PG)E2 synthesis using selective enzyme inhibitors. RESULTS—There was strong parietal cell COX-1 and COX-2 immunoreactivity in all sections and isolated cells, with macrophage and myofibroblast reactivity in some sections. Immunostaining was specifically abolished by antigen absorption. Western blot analysis confirmed COX-1 and 2 expression. COX-1 and COX-2 immunostaining was increased in Helicobacter pylori gastritis, particularly the mid glandular zone and lamina propria inflammatory cells. This was associated with increased ex vivo PGE2 synthesis (62.4 (13.5) pg/mg v 36.3 (15.5) pg/mg in uninflamed mucosa; p=0.017) which was significantly inhibited by COX-1 but not COX-2 inhibition. Increased COX-2 immunostaining in macrophages, endothelial cells, and myofibroblasts (with reduced epithelial expression) was seen at the rim of ulcers. CONCLUSION—COX-2, as well as COX-1, is expressed by normal human gastric mucosa and is increased at the rim of ulcers. Although both are increased with H pylori, COX-1 contributes more than COX-2 to gastric PGE2 production. Keywords: stomach; gastric mucosa; cyclooxygenases; Helicobacter pylori; ulceration; prostaglandins
Full Text
The Full Text of this article is available as a PDF (340.9 KB).
Figure 1 .
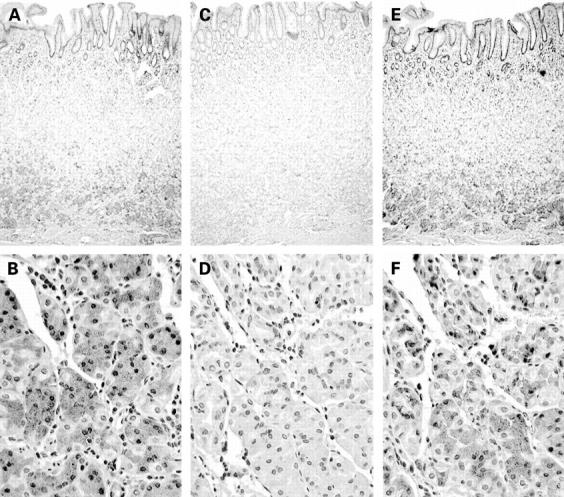
Immunohistochemistry of cyclooxygenase (COX)-1 and COX-2 enzymes in normal human gastric mucosa. Immunoperoxidase activity was positive in glandular epithelial cells when incubated with antibodies against COX-1 (A) and COX-2 (B), but negative when incubated with an anti-COX-1 antibody preabsorbed with purified COX-1 (C) and an anti-COX-2 antibody preabsorbed with purified COX-2 (D). Preincubation of COX-1 antibody with COX-2 antigen (E) and COX-2 antibody with COX-1 antigen (F) did not block staining. Original magnification: ×25 for A, C, E; ×160 for B, D, F (note, sections are not contiguous).
Figure 2 .
Immunohistochemical analysis of human gastric mucosa with antibodies to cyclooxygenase (COX)-1 (A) and human milk fat globule 2 (HMFG2) (B). Immunopositive cells are represented with morphological features of parietal cells (arrows). In cytospin preparations, cells with the morphological features of parietal cells demonstrated cytoplasmic immunoreactivity for HMFG2 (C), COX-1 (D), and COX-2 (E).
Figure 3 .
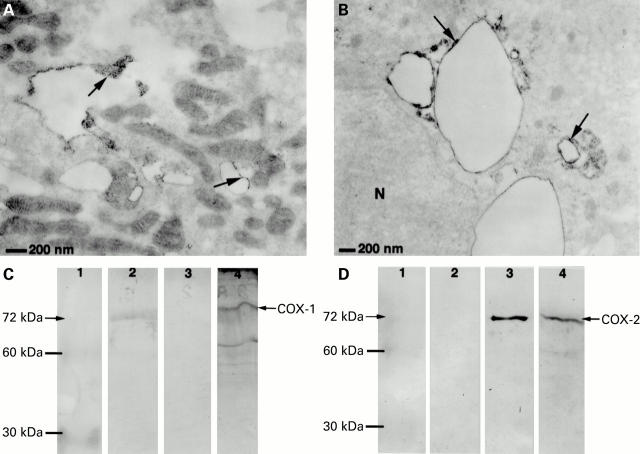
Electron photomicrography of human gastric glands stained with antibodies to cyclooxygenase (COX)-1 (A) and COX-2 (B). Immunoreactivity was localised to intracellular membrane structures (arrows), likely to be smooth endoplasmic reticulum or canicular membranes. (C, D) Western blot analysis of COX-1 and COX-2 proteins in human gastric epithelial cells. Cell lysate (10 µg, lane 4) was separated by sodium dodecyl sulphate-polyacrylamide gel electrophoresis, transferred to nitrocellulose membranes, and immunoblotted with (C) anti-COX-1 and (D) anti-COX-2 antibodies. Purified COX-1 and COX-2 standards (0.5 µg) were treated in the same way as the cell lysate and are shown on lane 2 and lane 3, respectively. Molecular weight marker for protein is shown on lane 1 with 60 kDa and 30 kDa bands present. An estimated 72 kDa protein band is seen on lanes with either COX standard or epithelial cells.
Figure 4 .
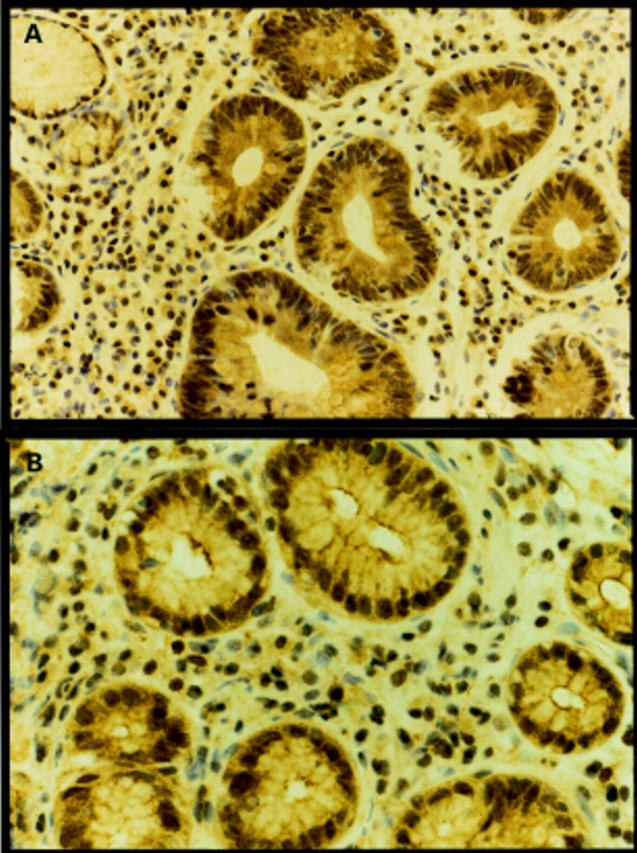
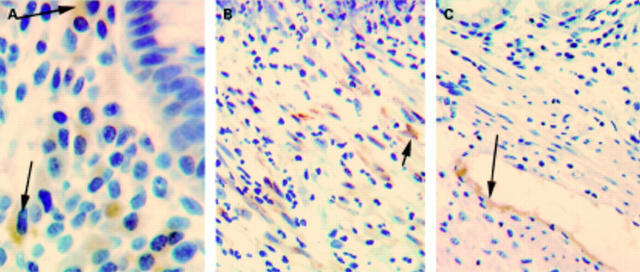
Immunohistochemistry of H pylori associated gastritis showed an increase in the relative intensity of staining of the proliferative zone of the epithelium with cyclooxygenase (COX)-1 (A) and COX-2 (B) antibodies. There was also an increase in the number of cells of the lamina propria which stained positively with COX-1 (A) and COX-2 (B) antibodies.
Figure 5 .
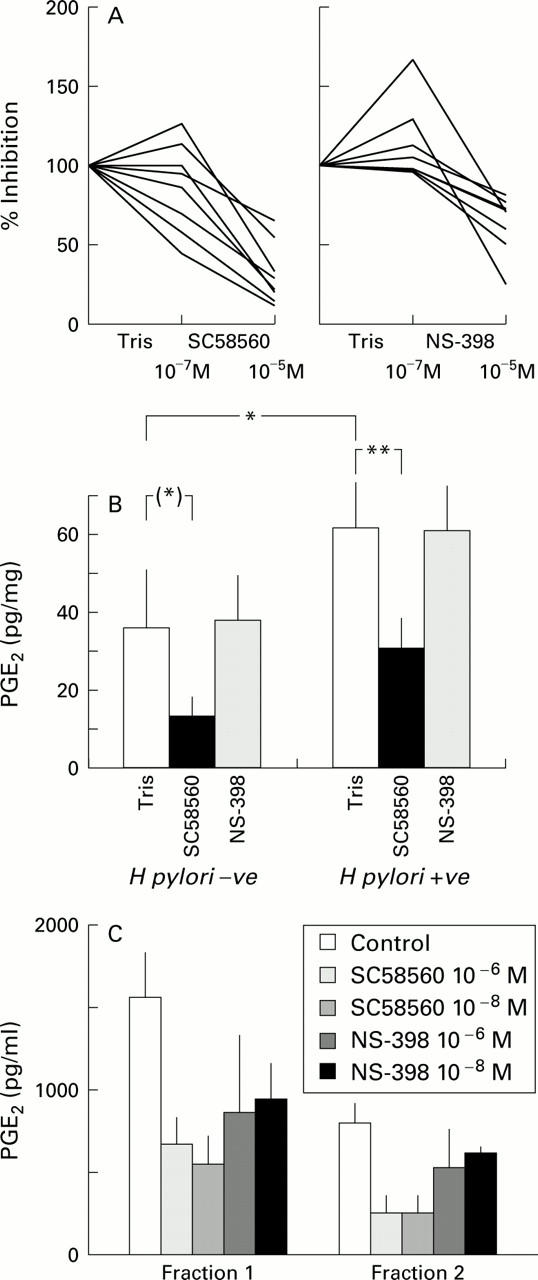
(A) Dose dependent inhibition of prostaglandin E2 (PGE2) production by cyclooxygenase (COX)-1 inhibitor SC58560 and COX-2 inhibitor NS-398 at concentrations of 10−7 M and 10−5 M. (B) COX-1 and COX-2 inhibition of ex vivo PGE2 production from whole biopsies by SC58560 and NS -398 at concentrations of 10−5 M in the presence or absence of H pylori. *p=0.017, **p=0.002, (*)p=0.13. (C) PGE2 production by parietal cell enriched fraction of human gastric epithelial cells (control) and its inhibition by SC58560 and NS-398 at concentrations of 10−6 M and 10−8 M.
Figure 6 .
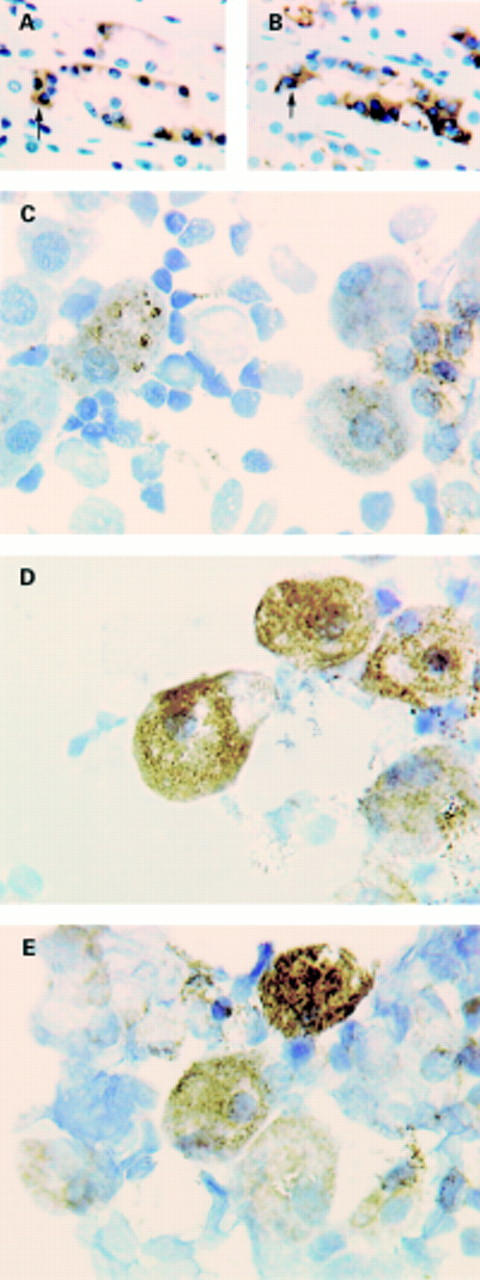
Immunohistochemistry of cyclooxygenase (COX)-2 enzyme in ulcerated human gastric mucosa. Immunoreactivity is seen on (A) macrophages in lamina propria proximal to the ulcer rim, (B) myofibroblasts at the ulcer base or granulation tissue, and (C) vascular endothelial cells in the submucosa next to the ulcer.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chan C. C., Boyce S., Brideau C., Ford-Hutchinson A. W., Gordon R., Guay D., Hill R. G., Li C. S., Mancini J., Penneton M. Pharmacology of a selective cyclooxygenase-2 inhibitor, L-745,337: a novel nonsteroidal anti-inflammatory agent with an ulcerogenic sparing effect in rat and nonhuman primate stomach. J Pharmacol Exp Ther. 1995 Sep;274(3):1531–1537. [PubMed] [Google Scholar]
- Choquet A., Magous R., Bali J. P. Gastric mucosal endogenous prostanoids are involved in the cellular regulation of acid secretion from isolated parietal cells. J Pharmacol Exp Ther. 1993 Sep;266(3):1306–1311. [PubMed] [Google Scholar]
- Coyne D. W., Nickols M., Bertrand W., Morrison A. R. Regulation of mesangial cell cyclooxygenase synthesis by cytokines and glucocorticoids. Am J Physiol. 1992 Jul;263(1 Pt 2):F97–102. doi: 10.1152/ajprenal.1992.263.1.F97. [DOI] [PubMed] [Google Scholar]
- DuBois R. N., Awad J., Morrow J., Roberts L. J., 2nd, Bishop P. R. Regulation of eicosanoid production and mitogenesis in rat intestinal epithelial cells by transforming growth factor-alpha and phorbol ester. J Clin Invest. 1994 Feb;93(2):493–498. doi: 10.1172/JCI116998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellows I. W., Bhaskar N. K., Hawkey C. J. Nature and time-course of piroxicam-induced injury to human gastric mucosa. Aliment Pharmacol Ther. 1989 Oct;3(5):481–488. doi: 10.1111/j.1365-2036.1989.tb00239.x. [DOI] [PubMed] [Google Scholar]
- Fu J. Y., Masferrer J. L., Seibert K., Raz A., Needleman P. The induction and suppression of prostaglandin H2 synthase (cyclooxygenase) in human monocytes. J Biol Chem. 1990 Oct 5;265(28):16737–16740. [PubMed] [Google Scholar]
- Gierse J. K., McDonald J. J., Hauser S. D., Rangwala S. H., Koboldt C. M., Seibert K. A single amino acid difference between cyclooxygenase-1 (COX-1) and -2 (COX-2) reverses the selectivity of COX-2 specific inhibitors. J Biol Chem. 1996 Jun 28;271(26):15810–15814. doi: 10.1074/jbc.271.26.15810. [DOI] [PubMed] [Google Scholar]
- Gretzer B., Ehrlich K., Maricic N., Lambrecht N., Respondek M., Peskar B. M. Selective cyclo-oxygenase-2 inhibitors and their influence on the protective effect of a mild irritant in the rat stomach. Br J Pharmacol. 1998 Mar;123(5):927–935. doi: 10.1038/sj.bjp.0701673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris R. C., McKanna J. A., Akai Y., Jacobson H. R., Dubois R. N., Breyer M. D. Cyclooxygenase-2 is associated with the macula densa of rat kidney and increases with salt restriction. J Clin Invest. 1994 Dec;94(6):2504–2510. doi: 10.1172/JCI117620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkey C. J., Hawthorne A. B., Hudson N., Cole A. T., Mahida Y. R., Daneshmend T. K. Separation of the impairment of haemostasis by aspirin from mucosal injury in the human stomach. Clin Sci (Lond) 1991 Oct;81(4):565–573. doi: 10.1042/cs0810565. [DOI] [PubMed] [Google Scholar]
- Hudson N., Balsitis M., Filipowicz F., Hawkey C. J. Effect of Helicobacter pylori colonisation on gastric mucosal eicosanoid synthesis in patients taking non-steroidal anti-inflammatory drugs. Gut. 1993 Jun;34(6):748–751. doi: 10.1136/gut.34.6.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull M. A., Thomson J. L., Hawkey C. J. Expression of cyclooxygenase 1 and 2 by human gastric endothelial cells. Gut. 1999 Oct;45(4):529–536. doi: 10.1136/gut.45.4.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iseki S. Immunocytochemical localization of cyclooxygenase-1 and cyclooxygenase-2 in the rat stomach. Histochem J. 1995 Apr;27(4):323–328. doi: 10.1007/BF00398975. [DOI] [PubMed] [Google Scholar]
- Kargman S. L., O'Neill G. P., Vickers P. J., Evans J. F., Mancini J. A., Jothy S. Expression of prostaglandin G/H synthase-1 and -2 protein in human colon cancer. Cancer Res. 1995 Jun 15;55(12):2556–2559. [PubMed] [Google Scholar]
- Kargman S., Charleson S., Cartwright M., Frank J., Riendeau D., Mancini J., Evans J., O'Neill G. Characterization of Prostaglandin G/H Synthase 1 and 2 in rat, dog, monkey, and human gastrointestinal tracts. Gastroenterology. 1996 Aug;111(2):445–454. doi: 10.1053/gast.1996.v111.pm8690211. [DOI] [PubMed] [Google Scholar]
- Klein T., Nüsing R. M., Pfeilschifter J., Ullrich V. Selective inhibition of cyclooxygenase 2. Biochem Pharmacol. 1994 Oct 18;48(8):1605–1610. doi: 10.1016/0006-2952(94)90205-4. [DOI] [PubMed] [Google Scholar]
- Kobayashi K., Higuchi K., Arakawa T., Matsumoto T., Nagura H. Effect of sofalcone on localization of 15-hydroxyprostaglandin dehydrogenase, an enzyme that metabolizes prostaglandin E2, in rat gastric mucosa: an immunohistochemical study. J Clin Gastroenterol. 1992;14 (Suppl 1):S39–S42. doi: 10.1097/00004836-199206001-00007. [DOI] [PubMed] [Google Scholar]
- Mahida Y. R., Beltinger J., Makh S., Göke M., Gray T., Podolsky D. K., Hawkey C. J. Adult human colonic subepithelial myofibroblasts express extracellular matrix proteins and cyclooxygenase-1 and -2. Am J Physiol. 1997 Dec;273(6 Pt 1):G1341–G1348. doi: 10.1152/ajpgi.1997.273.6.G1341. [DOI] [PubMed] [Google Scholar]
- Mahida Y. R., Galvin A. M., Gray T., Makh S., McAlindon M. E., Sewell H. F., Podolsky D. K. Migration of human intestinal lamina propria lymphocytes, macrophages and eosinophils following the loss of surface epithelial cells. Clin Exp Immunol. 1997 Aug;109(2):377–386. doi: 10.1046/j.1365-2249.1997.4481346.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno H., Sakamoto C., Matsuda K., Wada K., Uchida T., Noguchi H., Akamatsu T., Kasuga M. Induction of cyclooxygenase 2 in gastric mucosal lesions and its inhibition by the specific antagonist delays healing in mice. Gastroenterology. 1997 Feb;112(2):387–397. doi: 10.1053/gast.1997.v112.pm9024292. [DOI] [PubMed] [Google Scholar]
- Morita I., Schindler M., Regier M. K., Otto J. C., Hori T., DeWitt D. L., Smith W. L. Different intracellular locations for prostaglandin endoperoxide H synthase-1 and -2. J Biol Chem. 1995 May 5;270(18):10902–10908. doi: 10.1074/jbc.270.18.10902. [DOI] [PubMed] [Google Scholar]
- Nakamura M., Oda M., Inoue J., Ito T., Akiba Y., Kitajima M., Tsuchiya M., Ishii H. Plasticity of myofibroblasts appearing in granulation tissues after acetic acid treatment. Effect of bFGF. Dig Dis Sci. 1995 Nov;40(11):2477–2480. doi: 10.1007/BF02063259. [DOI] [PubMed] [Google Scholar]
- Nakamura M., Oda M., Inoue J., Nishizaki Y., Tsuchiya M. Roles of muscularis mucosae and myofibroblasts in the healing process of acetic acid-induced ulcer. J Clin Gastroenterol. 1990;12 (Suppl 1):S39–S47. doi: 10.1097/00004836-199001001-00008. [DOI] [PubMed] [Google Scholar]
- O'Neill G. P., Ford-Hutchinson A. W. Expression of mRNA for cyclooxygenase-1 and cyclooxygenase-2 in human tissues. FEBS Lett. 1993 Sep 13;330(2):156–160. doi: 10.1016/0014-5793(93)80263-t. [DOI] [PubMed] [Google Scholar]
- O'Sullivan M. G., Huggins E. M., Jr, Meade E. A., DeWitt D. L., McCall C. E. Lipopolysaccharide induces prostaglandin H synthase-2 in alveolar macrophages. Biochem Biophys Res Commun. 1992 Sep 16;187(2):1123–1127. doi: 10.1016/0006-291x(92)91313-f. [DOI] [PubMed] [Google Scholar]
- Ota S., Hata Y., Hiraishi H., Mutoh H., Terano A., Sugimoto T. The effects of acid secretagogues on protective agents of gastric cells from adult rabbits in vitro. J Clin Gastroenterol. 1992;14 (Suppl 1):S156–S161. doi: 10.1097/00004836-199206001-00028. [DOI] [PubMed] [Google Scholar]
- Ota S., Razandi M., Krause W., Terano A., Hiraishi H., Ivey K. J. Prostaglandin E2 output by isolated rat gastric parietal cells and non-parietal epithelial cells. Prostaglandins. 1988 Nov;36(5):589–600. doi: 10.1016/0090-6980(88)90005-6. [DOI] [PubMed] [Google Scholar]
- Payne N. A., Gerber J. G. Prostaglandin E2 and [14C]arachidonic acid release by carbachol in the isolated canine parietal cell. J Pharmacol Exp Ther. 1987 Nov;243(2):511–516. [PubMed] [Google Scholar]
- Regier M. K., Otto J. C., DeWitt D. L., Smith W. L. Localization of prostaglandin endoperoxide synthase-1 to the endoplasmic reticulum and nuclear envelope is independent of its C-terminal tetrapeptide-PTEL. Arch Biochem Biophys. 1995 Mar 10;317(2):457–463. doi: 10.1006/abbi.1995.1188. [DOI] [PubMed] [Google Scholar]
- Reiger M. K., DeWitt D. L., Schindler M. S., Smith W. L. Subcellular localization of prostaglandin endoperoxide synthase-2 in murine 3T3 cells. Arch Biochem Biophys. 1993 Mar;301(2):439–444. doi: 10.1006/abbi.1993.1168. [DOI] [PubMed] [Google Scholar]
- Robert A., Nezamis J. E., Lancaster C., Hanchar A. J. Cytoprotection by prostaglandins in rats. Prevention of gastric necrosis produced by alcohol, HCl, NaOH, hypertonic NaCl, and thermal injury. Gastroenterology. 1979 Sep;77(3):433–443. [PubMed] [Google Scholar]
- Ryan G. B., Cliff W. J., Gabbiani G., Irlé C., Montandon D., Statkov P. R., Majno G. Myofibroblasts in human granulation tissue. Hum Pathol. 1974 Jan;5(1):55–67. doi: 10.1016/s0046-8177(74)80100-0. [DOI] [PubMed] [Google Scholar]
- Sawaoka H., Kawano S., Tsuji S., Tsuji M., Sun W., Gunawan E. S., Hori M. Helicobacter pylori infection induces cyclooxygenase-2 expression in human gastric mucosa. Prostaglandins Leukot Essent Fatty Acids. 1998 Nov;59(5):313–316. doi: 10.1016/s0952-3278(98)90079-5. [DOI] [PubMed] [Google Scholar]
- Schepp W., Miederer S. E., Ruoff H. J., Wulfhekel U. Isolierte menschliche Magenschleimhautzellen--Untersuchungen physiologischer und pharmakologischer Regulationsmechanismen. Klin Wochenschr. 1986 Jan 2;64(1):15–22. doi: 10.1007/BF01721576. [DOI] [PubMed] [Google Scholar]
- Schmassmann A., Peskar B. M., Stettler C., Netzer P., Stroff T., Flogerzi B., Halter F. Effects of inhibition of prostaglandin endoperoxide synthase-2 in chronic gastro-intestinal ulcer models in rats. Br J Pharmacol. 1998 Mar;123(5):795–804. doi: 10.1038/sj.bjp.0701672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seibert K., Zhang Y., Leahy K., Hauser S., Masferrer J., Perkins W., Lee L., Isakson P. Pharmacological and biochemical demonstration of the role of cyclooxygenase 2 in inflammation and pain. Proc Natl Acad Sci U S A. 1994 Dec 6;91(25):12013–12017. doi: 10.1073/pnas.91.25.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoglund M. L., Gerber J. G., Murphy R. C., Nies A. S. Prostaglandin production by intact isolated gastric parietal cells. Eur J Pharmacol. 1980 Aug 22;66(1):145–148. doi: 10.1016/0014-2999(80)90309-x. [DOI] [PubMed] [Google Scholar]
- Skoglund M. L., Nies A. S., Gerber J. G. Inhibition of acid secretion in isolated canine parietal cells by prostaglandins. J Pharmacol Exp Ther. 1982 Feb;220(2):371–374. [PubMed] [Google Scholar]
- Smith C. J., Zhang Y., Koboldt C. M., Muhammad J., Zweifel B. S., Shaffer A., Talley J. J., Masferrer J. L., Seibert K., Isakson P. C. Pharmacological analysis of cyclooxygenase-1 in inflammation. Proc Natl Acad Sci U S A. 1998 Oct 27;95(22):13313–13318. doi: 10.1073/pnas.95.22.13313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarnawski A., Brzozowski T., Sarfeh I. J., Krause W. J., Ulich T. R., Gergely H., Hollander D. Prostaglandin protection of human isolated gastric glands against indomethacin and ethanol injury. Evidence for direct cellular action of prostaglandin. J Clin Invest. 1988 Apr;81(4):1081–1089. doi: 10.1172/JCI113420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uribe A., Alam M., Midtvedt T. E2 prostaglandins modulate cell proliferation in the small intestinal epithelium of the rat. Digestion. 1992;52(3-4):157–164. doi: 10.1159/000200948. [DOI] [PubMed] [Google Scholar]
- Vane J. R. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol. 1971 Jun 23;231(25):232–235. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]
- Vane J. Towards a better aspirin. Nature. 1994 Jan 20;367(6460):215–216. doi: 10.1038/367215a0. [DOI] [PubMed] [Google Scholar]
- Walker M. M., Smolka A., Waller J. M., Evans D. J. Identification of parietal cells in gastric body mucosa with HMFG-2 monoclonal antibody. J Clin Pathol. 1995 Sep;48(9):832–834. doi: 10.1136/jcp.48.9.832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace J. L., Bak A., McKnight W., Asfaha S., Sharkey K. A., MacNaughton W. K. Cyclooxygenase 1 contributes to inflammatory responses in rats and mice: implications for gastrointestinal toxicity. Gastroenterology. 1998 Jul;115(1):101–109. doi: 10.1016/s0016-5085(98)70370-1. [DOI] [PubMed] [Google Scholar]
- Whittle B. J. Temporal relationship between cyclooxygenase inhibition, as measured by prostacyclin biosynthesis, and the gastrointestinal damage induced by indomethacin in the rat. Gastroenterology. 1981 Jan;80(1):94–98. [PubMed] [Google Scholar]