Abstract
BACKGROUND/AIMS—C-type natriuretic peptide (CNP), the third member of the natriuretic peptide family, is considered to be involved in the regulation of vascular tone. Furthermore, the recent demonstration of CNP in human kidney and urine may indicate a role for CNP in fluid and electrolyte homeostasis. Therefore, the aim of the present study was to investigate the possible role of CNP in renal function disturbances in patients with cirrhosis of the liver. METHODS—Peripheral venous and urinary concentrations of CNP were determined in samples from 11 healthy controls, 20 cirrhotic patients with normal renal function (creatinine clearance 117 (8) ml/min), and 20 cirrhotic patients with impaired renal function (creatinine clearance 35 (4) ml/min). In a second protocol, arterial and renal venous plasma concentrations of CNP were determined in 37 patients with cirrhosis of the liver to estimate renal extraction ratios of CNP. A sensitive and specific radioimmunoassay was applied after solid phase extraction of samples. RESULTS—Plasma CNP was lower in cirrhotic patients with normal and impaired renal function than in controls (3.0 (0.4) and 2.7 (0.2) v 4.2 (0.4) pg/ml, respectively; p< 0.05; mean (SEM)). In contrast, urinary CNP was higher in patients with impaired renal function compared with those with normal renal function and healthy controls (47.2 (7.4) v 20.8 (1.9) and 17.0 (3.0) ng CNP/g creatinine, respectively; p<0.05). Urinary CNP was found to be inversely related to urinary sodium excretion in cirrhotic patients (r=−0.56; p<0.01). No differences were observed between arterial and renal venous concentrations of CNP in cirrhosis (2.4 (0.2) v 2.4 (0.2) pg/ml). In cirrhotic patients with hepatorenal syndrome or refractory ascites (n=5), urinary CNP decreased from 132 (59) to 38 (7) ng/g creatinine (p<0.05) one week after either ornipressin infusion or insertion of a transjugular intrahepatic portosystemic shunt together with an increase in urinary sodium excretion from 27 (17) to 90 (34) mmol/24 hours. CONCLUSIONS—Increased urinary CNP in cirrhotic patients in the absence of renal arteriovenous concentration gradients suggests enhanced renal CNP production in cirrhosis. Furthermore, an inverse relation between urinary CNP and urinary sodium excretion suggests a role for this peptide in renal sodium handling in patients with cirrhosis. Keywords: C-type natriuretic peptide; renal function; natriuresis; cirrhosis
Full Text
The Full Text of this article is available as a PDF (140.8 KB).
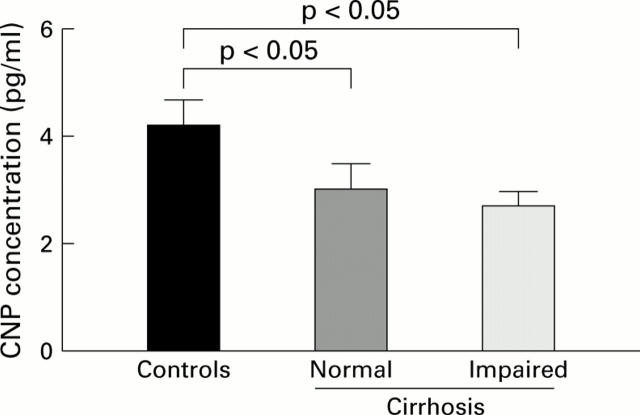
Figure 1 .
Circulating plasma concentrations of C-type natriuretic peptide (CNP) in control subjects (n=11) and in cirrhotic patients with normal (n=20) or impaired renal function (n=20). Data are mean (SEM).
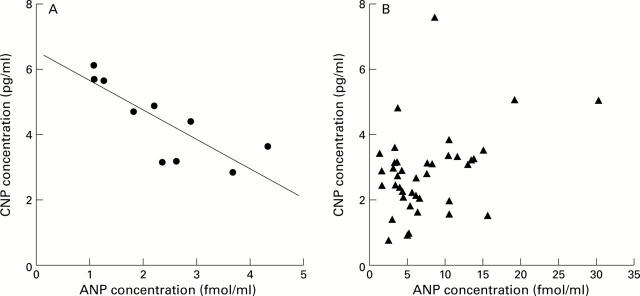
Figure 2 .
Circulating plasma concentrations of C-type natriuretic peptide (CNP) were inversely correlated with circulating atrial natriuretic peptide (ANP) plasma concentrations in healthy controls (r=−0.80; p=0.005; ANP plasma concentration only available in 10 subjects; A). No such relationship was observed in cirrhotic patients with normal or impaired renal function (B).
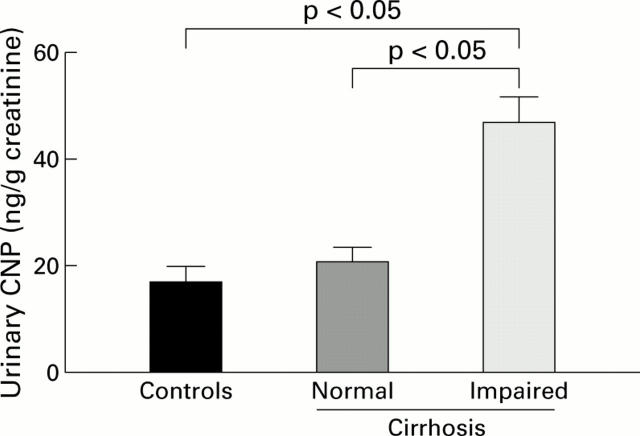
Figure 3 .
Urinary excretion of C-type natriuretic peptide (CNP) was significantly increased in cirrhotic patients with impaired renal function (n=20) compared with those with normal renal function (n=20) or healthy controls (n=11). Data are mean (SEM).
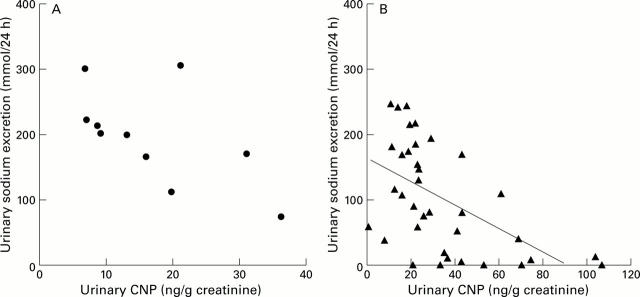
Figure 4 .
Urinary excretion of C-type natriuretic peptide (CNP) was inversely correlated with urinary sodium excretion in cirrhotic patients (r=−0.56; p<0.01; B), but not in control subjects (A).
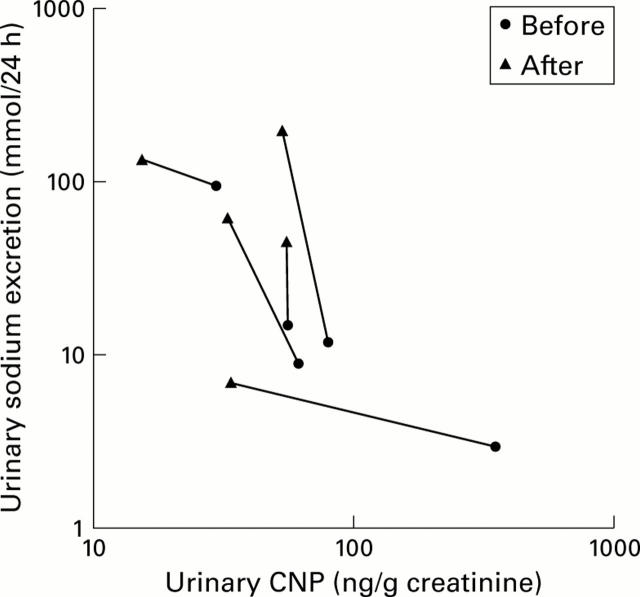
Figure 5 .
Changes in urinary C-type natriuretic peptide (CNP) and natriuresis in five patients following therapeutic interventions. Due to the skewed distribution of the values, both variables are presented on a logarithmic scale.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angeli P., Jiménez W., Arroyo V., Mackenzie H. S., Zhang P. L., Clària J., Rivera F., Brenner B. M., Rodés J. Renal effects of natriuretic peptide receptor blockade in cirrhotic rats with ascites. Hepatology. 1994 Oct;20(4 Pt 1):948–954. doi: 10.1002/hep.1840200425. [DOI] [PubMed] [Google Scholar]
- Barletta G., Lazzeri C., Vecchiarino S., Del Bene R., Messeri G., Dello Sbarba A., Mannelli M., La Villa G. Low-dose C-type natriuretic peptide does not affect cardiac and renal function in humans. Hypertension. 1998 Mar;31(3):802–808. doi: 10.1161/01.hyp.31.3.802. [DOI] [PubMed] [Google Scholar]
- Dean A. D., Vehaskari V. M., Greenwald J. E. Synthesis and localization of C-type natriuretic peptide in mammalian kidney. Am J Physiol. 1994 Mar;266(3 Pt 2):F491–F496. doi: 10.1152/ajprenal.1994.266.3.F491. [DOI] [PubMed] [Google Scholar]
- Forssmann W. G., Richter R., Meyer M. The endocrine heart and natriuretic peptides: histochemistry, cell biology, and functional aspects of the renal urodilatin system. Histochem Cell Biol. 1998 Oct;110(4):335–357. doi: 10.1007/s004180050295. [DOI] [PubMed] [Google Scholar]
- Gerbes A. L., Arendt R. M., Paumgartner G. Atrial natriuretic factor. Possible implications in liver disease. J Hepatol. 1987 Aug;5(1):123–132. doi: 10.1016/s0168-8278(87)80070-3. [DOI] [PubMed] [Google Scholar]
- Gerbes A. L., Gülberg V., Waggershauser T., Holl J., Reiser M. Renal effects of transjugular intrahepatic portosystemic shunt in cirrhosis: comparison of patients with ascites, with refractory ascites, or without ascites. Hepatology. 1998 Sep;28(3):683–688. doi: 10.1002/hep.510280313. [DOI] [PubMed] [Google Scholar]
- Gerbes A. L., Møller S., Gülberg V., Henriksen J. H. Endothelin-1 and -3 plasma concentrations in patients with cirrhosis: role of splanchnic and renal passage and liver function. Hepatology. 1995 Mar;21(3):735–739. [PubMed] [Google Scholar]
- Gerbes A. L. The role of atrial natriuretic peptide (ANP) in chronic liver disease. Pharmacol Ther. 1993 Jun;58(3):381–390. doi: 10.1016/0163-7258(93)90028-c. [DOI] [PubMed] [Google Scholar]
- Gerbes A. L., Wernze H., Arendt R. M., Riedel A., Sauerbruch T., Paumgartner G. Atrial natriuretic factor and renin-aldosterone in volume regulation of patients with cirrhosis. Hepatology. 1989 Mar;9(3):417–422. doi: 10.1002/hep.1840090312. [DOI] [PubMed] [Google Scholar]
- Guevara M., Ginès P., Bandi J. C., Gilabert R., Sort P., Jiménez W., Garcia-Pagan J. C., Bosch J., Arroyo V., Rodés J. Transjugular intrahepatic portosystemic shunt in hepatorenal syndrome: effects on renal function and vasoactive systems. Hepatology. 1998 Aug;28(2):416–422. doi: 10.1002/hep.510280219. [DOI] [PubMed] [Google Scholar]
- Guevara M., Ginès P., Fernández-Esparrach G., Sort P., Salmerón J. M., Jiménez W., Arroyo V., Rodés J. Reversibility of hepatorenal syndrome by prolonged administration of ornipressin and plasma volume expansion. Hepatology. 1998 Jan;27(1):35–41. doi: 10.1002/hep.510270107. [DOI] [PubMed] [Google Scholar]
- Gunning M. E., Brenner B. M. Natriuretic peptides and the kidney: current concepts. Kidney Int Suppl. 1992 Oct;38:S127–S133. [PubMed] [Google Scholar]
- Gülberg V., Bilzer M., Gerbes A. L. Long-term therapy and retreatment of hepatorenal syndrome type 1 with ornipressin and dopamine. Hepatology. 1999 Oct;30(4):870–875. doi: 10.1002/hep.510300430. [DOI] [PubMed] [Google Scholar]
- Hunt P. J., Richards A. M., Espiner E. A., Nicholls M. G., Yandle T. G. Bioactivity and metabolism of C-type natriuretic peptide in normal man. J Clin Endocrinol Metab. 1994 Jun;78(6):1428–1435. doi: 10.1210/jcem.78.6.8200946. [DOI] [PubMed] [Google Scholar]
- Koller K. J., Goeddel D. V. Molecular biology of the natriuretic peptides and their receptors. Circulation. 1992 Oct;86(4):1081–1088. doi: 10.1161/01.cir.86.4.1081. [DOI] [PubMed] [Google Scholar]
- Komeichi H., Moreau R., Cailmail S., Gaudin C., Lebrec D. Blunted natriuresis and abnormal systemic hemodynamic responses to C-type and brain natriuretic peptides in rats with cirrhosis. J Hepatol. 1995 Mar;22(3):319–325. doi: 10.1016/0168-8278(95)80285-1. [DOI] [PubMed] [Google Scholar]
- La Villa G., Romanelli R. G., Casini Raggi V., Tosti-Guerra C., De Feo M. L., Marra F., Laffi G., Gentilini P. Plasma levels of brain natriuretic peptide in patients with cirrhosis. Hepatology. 1992 Jul;16(1):156–161. doi: 10.1002/hep.1840160126. [DOI] [PubMed] [Google Scholar]
- Mattingly M. T., Brandt R. R., Heublein D. M., Wei C. M., Nir A., Burnett J. C., Jr Presence of C-type natriuretic peptide in human kidney and urine. Kidney Int. 1994 Sep;46(3):744–747. doi: 10.1038/ki.1994.329. [DOI] [PubMed] [Google Scholar]
- Moore K., Wendon J., Frazer M., Karani J., Williams R., Badr K. Plasma endothelin immunoreactivity in liver disease and the hepatorenal syndrome. N Engl J Med. 1992 Dec 17;327(25):1774–1778. doi: 10.1056/NEJM199212173272502. [DOI] [PubMed] [Google Scholar]
- Saló J., Jiménez W., Kuhn M., Ginès A., Ginès P., Fernández-Esparrach G., Angeli P., Clària J., Bataller R., Arroyo V. Urinary excretion of urodilatin in patients with cirrhosis. Hepatology. 1996 Dec;24(6):1428–1432. doi: 10.1002/hep.510240621. [DOI] [PubMed] [Google Scholar]
- Schrier R. W., Arroyo V., Bernardi M., Epstein M., Henriksen J. H., Rodés J. Peripheral arterial vasodilation hypothesis: a proposal for the initiation of renal sodium and water retention in cirrhosis. Hepatology. 1988 Sep-Oct;8(5):1151–1157. doi: 10.1002/hep.1840080532. [DOI] [PubMed] [Google Scholar]
- Stingo A. J., Clavell A. L., Aarhus L. L., Burnett J. C., Jr Cardiovascular and renal actions of C-type natriuretic peptide. Am J Physiol. 1992 Jan;262(1 Pt 2):H308–H312. doi: 10.1152/ajpheart.1992.262.1.H308. [DOI] [PubMed] [Google Scholar]
- Terada Y., Tomita K., Nonoguchi H., Yang T., Marumo F. PCR localization of C-type natriuretic peptide and B-type receptor mRNAs in rat nephron segments. Am J Physiol. 1994 Aug;267(2 Pt 2):F215–F222. doi: 10.1152/ajprenal.1994.267.2.F215. [DOI] [PubMed] [Google Scholar]
- Vollmar A. M., Gerbes A. L., Nemer M., Schulz R. Detection of C-type natriuretic peptide (CNP) transcript in the rat heart and immune organs. Endocrinology. 1993 Apr;132(4):1872–1874. doi: 10.1210/endo.132.4.8462485. [DOI] [PubMed] [Google Scholar]
- Vollmar A. M., Paumgartner G., Gerbes A. L. Differential gene expression of the three natriuretic peptides and natriuretic peptide receptor subtypes in human liver. Gut. 1997 Jan;40(1):145–150. doi: 10.1136/gut.40.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner L., Skorecki K., Blendis L. M., Epstein M. Atrial natriuretic factor and liver disease. Hepatology. 1993 Mar;17(3):500–513. [PubMed] [Google Scholar]
- Wei C. M., Aarhus L. L., Miller V. M., Burnett J. C., Jr Action of C-type natriuretic peptide in isolated canine arteries and veins. Am J Physiol. 1993 Jan;264(1 Pt 2):H71–H73. doi: 10.1152/ajpheart.1993.264.1.H71. [DOI] [PubMed] [Google Scholar]