Abstract
BACKGROUND—Non-steroidal anti-inflammatory drugs (NSAIDs) have been reported to protect against the development of colon cancer. However, the mechanism(s) by which NSAIDs exert their effects is not clear. AIMS—The aim of this study was to examine the effects of NSAIDs on mRNA expression of tumour suppressor adenomatous polyposis coli (APC) gene in rat colon mucosa. METHODS—Starting at six weeks of age, three groups of rats (groups 1, 2, and 3) were treated with azoxymethane (AOM), a colon specific carcinogen, and another three groups (groups 4, 5, and 6) were not given AOM. Groups 2 and 3 were given 10 mg/kg of sulindac or etodolac, respectively, three times weekly during the experiment. Groups 4 and 5 were also given sulindac or etodolac, respectively, in the same manner as in groups 2 and 3. Group 6 (untreated control) was not given any agent (AOM or NSAIDs). At 10 weeks of age, preneoplastic lesions (aberrant crypt foci (ACF)) induced by AOM in the colon were counted, and the level of expression of APC mRNA in the colonic mucosa was estimated by the reverse transcription-competitive polymerase chain reaction method and northern blot analysis. RESULTS—Mean occurrence of ACF in rats in groups 2 and 3 was reduced to approximately 50% of that in group 1. The level of APC mRNA expression in group 1 (AOM alone) was lower than that in group 6 (untreated control) (p<0.05); however, levels of APC mRNA expression in groups 2, 3, 4, and 5, to which NSAIDs had been administered, were significantly increased compared with levels in groups 1 and 6 (p<0.01). CONCLUSIONS—Both sulindac and etodolac reduced the occurrence of ACF and induced an increase in APC mRNA in rat colon mucosa. Keywords: adenomatous polyposis coli; cyclooxygenase; non-steroidal anti-inflammatory drugs; aberrant crypt foci; colon; azoxymethane
Full Text
The Full Text of this article is available as a PDF (193.5 KB).
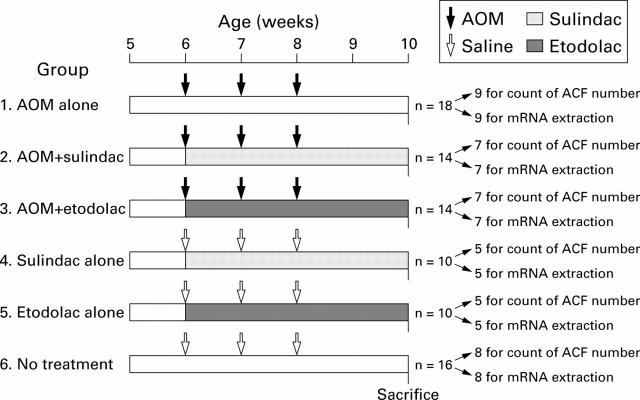
Figure 1 .
Experimental protocol. Azoxymethane (AOM) was given as 15 mg/kg by subcutaneous injection, saline as 0.3 ml subcutaneously, sulindac as 10 mg/kg in 5% gum arabic aqueous solution three times a week by oral savage, and etodolac as 10 mg/kg in 5% gum arabic aqueous solution three times a week by oral gavage.
Figure 2 .
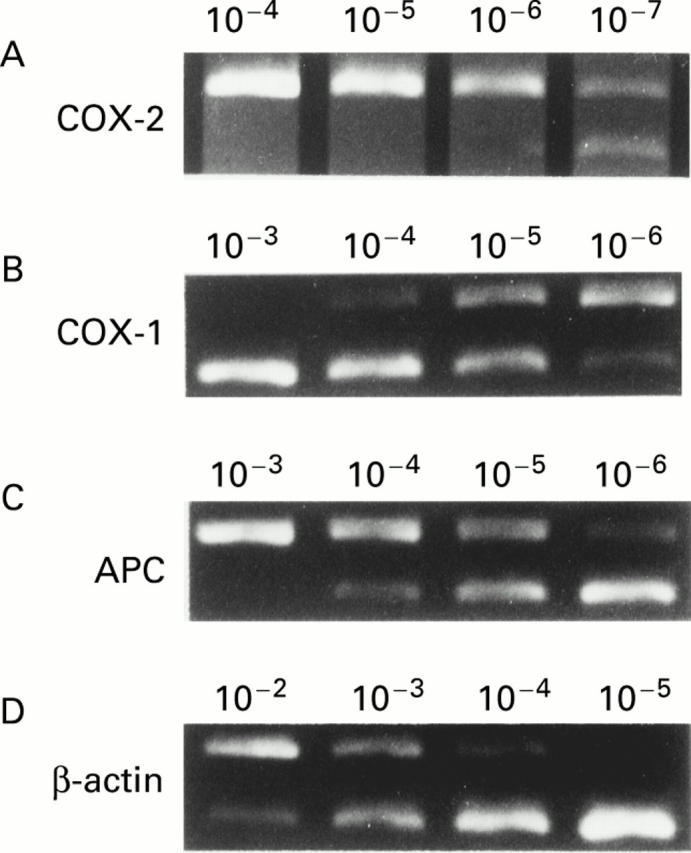
Analysis of cyclooxygenase (COX)-2, COX-1, adenomatous polyposis coli (APC), and β-actin mRNA expression. mRNA extracted from rat colonic tissue was reverse transcribed and amplified in the presence of different amounts of mimic DNA (lanes, from left to right: 10−4, 10−5, 10−6, 10−7 ng for COX-2; 10−3, 10−4, 10−5, 10−6 ng for COX-1 and APC; 10−2, 10−3, 10−4, 10−5 ng for β-actin). (A) COX-2 expression in colonic tissue of a representative rat in group 3. The level of COX-2 expression was 10−7 ng in this case. (B) COX-1 expression in the same rat as in (A). The level of target cDNA of COX-1 was 10−5 ng in this case. (C) APC expression in colonic tissue of a representative rat in group 2. The level of target cDNA of APC was 10−5 ng in this case. (D) β-actin expression in the same rat as in (C). The level of target cDNA of β-actin was 10−3 ng in this case. The level of β-actin expression was used as an internal control.
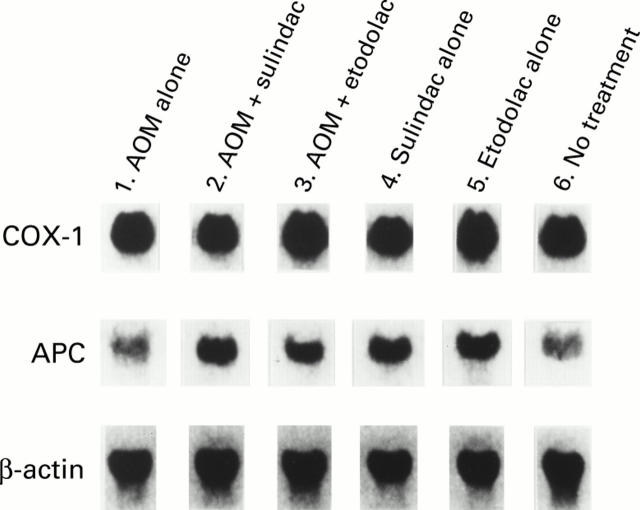
Figure 3 .
Northern blot analysis of cyclooxygenase (COX)-1 and adenomatous polyposis coli (APC) mRNAs from each group (groups 1, 2, 3, 4, 5, and 6). β-Actin expression was used as an internal control.
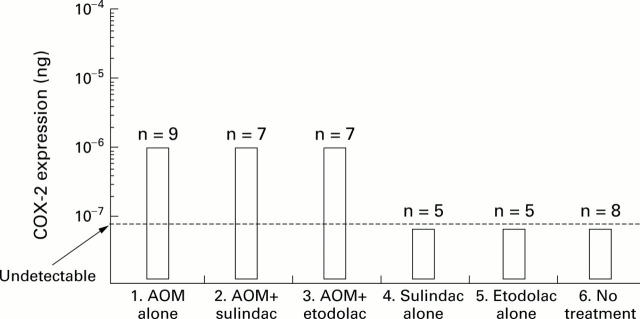
Figure 4 .
Levels of COX-2 mRNA expression measured by RT-competitive PCR. Data are shown as box plots: the box covers all values (including minimum and maximum values). The broken line indicates the limit of detection for COX-2 mRNA expression by RT-PCR. Levels of COX-2 expression in each case were normalised to that of β-actin mRNA (10−3 ng of mimic DNA). There was a tendency towards increased levels of COX-2 expression in the groups administered azoxymethane (AOM). However, levels were not significantly different between groups.
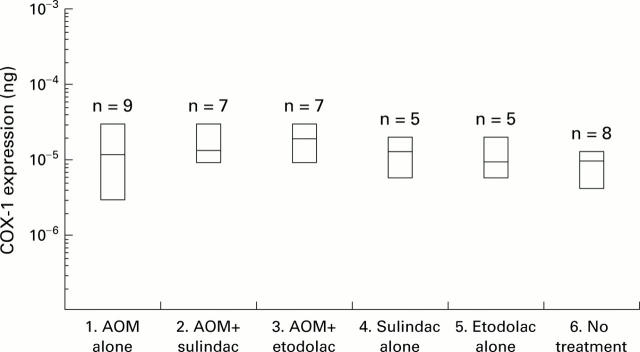
Figure 5 .
Levels of COX-1 expression measured by RT-competitive PCR. Data are shown as box plots: the box covers all values (including minimum and maximum values), and the central continuous line in each box represents the mean. Levels of COX-1 expression in each case were normalised to that of β-actin mRNA (10−3 ng of mimic DNA). There was no difference in COX-1 mRNA expression levels between groups. AOM, azoxymethane.
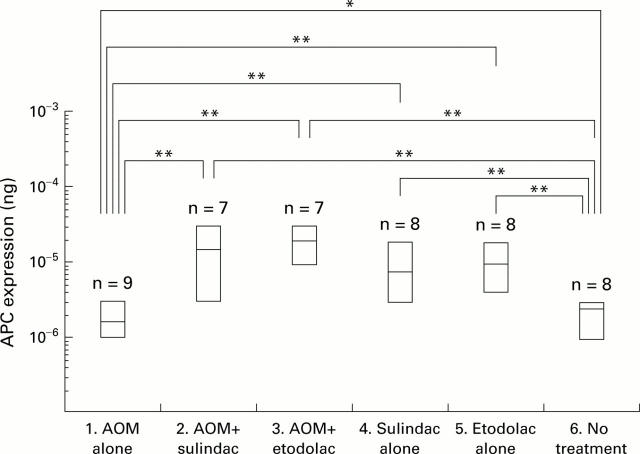
Figure 6 .
Levels of adenomatous polyposis coli (APC) expression measured by RT-competitive PCR. Data are shown as box plots: the box covers all values (including minimum and maximum values), and the central continuous line in each box represents the mean. Levels of APC expression in each case were normalised to that of β-actin mRNA (10−3 ng of mimic DNA). APC mRNA expression level in group 1 (azoxymethane (AOM) alone) was significantly lower than that in group 6 (untreated control) (p<0.05). Both sulindac and etodolac caused an increase in APC mRNA in the rat colonic mucosa (groups 2, 3, 4, and 5) (p<0.01). *p<0.05; **p<0.01.
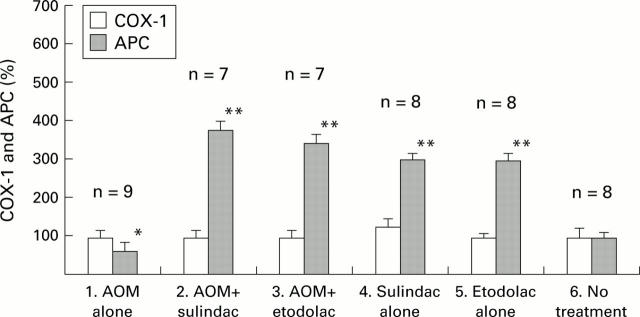
Figure 7 .
COX-1 and APC mRNA expression measured by northern blot analysis. mRNA levels were measured by northern blot analysis as described in materials and methods. Values are expressed as the ratio (%) to the average of those of group 6 (no treatment). Levels of APC mRNA expression in group 1 were lower than those in group 6 (p<0.05). Levels of APC mRNA expression in groups 2, 3, 4, and 5 were significantly increased compared with those in group 6 (p<0.01). *p<0.05;**p<0.01.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bodmer W. F., Bailey C. J., Bodmer J., Bussey H. J., Ellis A., Gorman P., Lucibello F. C., Murday V. A., Rider S. H., Scambler P. Localization of the gene for familial adenomatous polyposis on chromosome 5. Nature. 1987 Aug 13;328(6131):614–616. doi: 10.1038/328614a0. [DOI] [PubMed] [Google Scholar]
- Carmichael J., Shankel S. W. Effects of nonsteroidal anti-inflammatory drugs on prostaglandins and renal function. Am J Med. 1985 Jun;78(6 Pt 1):992–1000. doi: 10.1016/0002-9343(85)90223-2. [DOI] [PubMed] [Google Scholar]
- Celi F. S., Cohen M. M., Antonarakis S. E., Wertheimer E., Roth J., Shuldiner A. R. Determination of gene dosage by a quantitative adaptation of the polymerase chain reaction (gd-PCR): rapid detection of deletions and duplications of gene sequences. Genomics. 1994 May 15;21(2):304–310. doi: 10.1006/geno.1994.1270. [DOI] [PubMed] [Google Scholar]
- Corwin H. L., Bonventre J. V. Renal insufficiency associated with nonsteroidal anti-inflammatory agents. Am J Kidney Dis. 1984 Sep;4(2):147–152. doi: 10.1016/s0272-6386(84)80063-3. [DOI] [PubMed] [Google Scholar]
- De Filippo C., Caderni G., Bazzicalupo M., Briani C., Giannini A., Fazi M., Dolara P. Mutations of the Apc gene in experimental colorectal carcinogenesis induced by azoxymethane in F344 rats. Br J Cancer. 1998 Jun;77(12):2148–2151. doi: 10.1038/bjc.1998.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhart C. E., Coffey R. J., Radhika A., Giardiello F. M., Ferrenbach S., DuBois R. N. Up-regulation of cyclooxygenase 2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology. 1994 Oct;107(4):1183–1188. doi: 10.1016/0016-5085(94)90246-1. [DOI] [PubMed] [Google Scholar]
- Erdman S. H., Wu H. D., Hixson L. J., Ahnen D. J., Gerner E. W. Assessment of mutations in Ki-ras and p53 in colon cancers from azoxymethane- and dimethylhydrazine-treated rats. Mol Carcinog. 1997 Jun;19(2):137–144. doi: 10.1002/(sici)1098-2744(199707)19:2<137::aid-mc8>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Glaser K., Sung M. L., O'Neill K., Belfast M., Hartman D., Carlson R., Kreft A., Kubrak D., Hsiao C. L., Weichman B. Etodolac selectively inhibits human prostaglandin G/H synthase 2 (PGHS-2) versus human PGHS-1. Eur J Pharmacol. 1995 Jul 25;281(1):107–111. doi: 10.1016/0014-2999(95)00302-2. [DOI] [PubMed] [Google Scholar]
- Gustafson-Svärd C., Lilja I., Hallbök O., Sjödahl R. Cyclooxygenase-1 and cyclooxygenase-2 gene expression in human colorectal adenocarcinomas and in azoxymethane induced colonic tumours in rats. Gut. 1996 Jan;38(1):79–84. doi: 10.1136/gut.38.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He T. C., Sparks A. B., Rago C., Hermeking H., Zawel L., da Costa L. T., Morin P. J., Vogelstein B., Kinzler K. W. Identification of c-MYC as a target of the APC pathway. Science. 1998 Sep 4;281(5382):1509–1512. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- Hixson L. J., Earnest D. L., Fennerty M. B., Sampliner R. E. NSAID effect on sporadic colon polyps. Am J Gastroenterol. 1993 Oct;88(10):1652–1656. [PubMed] [Google Scholar]
- Ilyas M., Tomlinson I. P. The interactions of APC, E-cadherin and beta-catenin in tumour development and progression. J Pathol. 1997 Jun;182(2):128–137. doi: 10.1002/(SICI)1096-9896(199706)182:2<128::AID-PATH839>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Inomata M., Ochiai A., Akimoto S., Kitano S., Hirohashi S. Alteration of beta-catenin expression in colonic epithelial cells of familial adenomatous polyposis patients. Cancer Res. 1996 May 1;56(9):2213–2217. [PubMed] [Google Scholar]
- Kargman S. L., O'Neill G. P., Vickers P. J., Evans J. F., Mancini J. A., Jothy S. Expression of prostaglandin G/H synthase-1 and -2 protein in human colon cancer. Cancer Res. 1995 Jun 15;55(12):2556–2559. [PubMed] [Google Scholar]
- Kawamori T., Tanaka T., Kojima T., Suzui M., Ohnishi M., Mori H. Suppression of azoxymethane-induced rat colon aberrant crypt foci by dietary protocatechuic acid. Jpn J Cancer Res. 1994 Jul;85(7):686–691. doi: 10.1111/j.1349-7006.1994.tb02415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamori T., Tanaka T., Ohnishi M., Hirose Y., Nakamura Y., Satoh K., Hara A., Mori H. Chemoprevention of azoxymethane-induced colon carcinogenesis by dietary feeding of S-methyl methane thiosulfonate in male F344 rats. Cancer Res. 1995 Sep 15;55(18):4053–4058. [PubMed] [Google Scholar]
- Kishimoto Y., Wada K., Nakamoto K., Ashida K., Kamisaki Y., Kawasaki H., Itoh T. Quantitative analysis of cyclooxygenase-2 gene expression on acute gastric injury induced by ischemia-reperfusion in rats. Life Sci. 1997;60(8):PL127–PL133. doi: 10.1016/s0024-3205(96)00694-7. [DOI] [PubMed] [Google Scholar]
- Kopp E., Ghosh S. Inhibition of NF-kappa B by sodium salicylate and aspirin. Science. 1994 Aug 12;265(5174):956–959. doi: 10.1126/science.8052854. [DOI] [PubMed] [Google Scholar]
- Labayle D., Fischer D., Vielh P., Drouhin F., Pariente A., Bories C., Duhamel O., Trousset M., Attali P. Sulindac causes regression of rectal polyps in familial adenomatous polyposis. Gastroenterology. 1991 Sep;101(3):635–639. doi: 10.1016/0016-5085(91)90519-q. [DOI] [PubMed] [Google Scholar]
- Ladenheim J., Garcia G., Titzer D., Herzenberg H., Lavori P., Edson R., Omary M. B. Effect of sulindac on sporadic colonic polyps. Gastroenterology. 1995 Apr;108(4):1083–1087. doi: 10.1016/0016-5085(95)90206-6. [DOI] [PubMed] [Google Scholar]
- Lehmann J. M., Lenhard J. M., Oliver B. B., Ringold G. M., Kliewer S. A. Peroxisome proliferator-activated receptors alpha and gamma are activated by indomethacin and other non-steroidal anti-inflammatory drugs. J Biol Chem. 1997 Feb 7;272(6):3406–3410. doi: 10.1074/jbc.272.6.3406. [DOI] [PubMed] [Google Scholar]
- McLellan E. A., Bird R. P. Aberrant crypts: potential preneoplastic lesions in the murine colon. Cancer Res. 1988 Nov 1;48(21):6187–6192. [PubMed] [Google Scholar]
- Morin P. J., Sparks A. B., Korinek V., Barker N., Clevers H., Vogelstein B., Kinzler K. W. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science. 1997 Mar 21;275(5307):1787–1790. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- Munemitsu S., Albert I., Souza B., Rubinfeld B., Polakis P. Regulation of intracellular beta-catenin levels by the adenomatous polyposis coli (APC) tumor-suppressor protein. Proc Natl Acad Sci U S A. 1995 Mar 28;92(7):3046–3050. doi: 10.1073/pnas.92.7.3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima M., Dinchuk J. E., Kargman S. L., Oshima H., Hancock B., Kwong E., Trzaskos J. M., Evans J. F., Taketo M. M. Suppression of intestinal polyposis in Apc delta716 knockout mice by inhibition of cyclooxygenase 2 (COX-2). Cell. 1996 Nov 29;87(5):803–809. doi: 10.1016/s0092-8674(00)81988-1. [DOI] [PubMed] [Google Scholar]
- Park H. S., Goodlad R. A., Wright N. A. The incidence of aberrant crypt foci and colonic carcinoma in dimethylhydrazine-treated rats varies in a site-specific manner and depends on tumor histology. Cancer Res. 1997 Oct 15;57(20):4507–4510. [PubMed] [Google Scholar]
- Pereira M. A., Barnes L. H., Rassman V. L., Kelloff G. V., Steele V. E. Use of azoxymethane-induced foci of aberrant crypts in rat colon to identify potential cancer chemopreventive agents. Carcinogenesis. 1994 May;15(5):1049–1054. doi: 10.1093/carcin/15.5.1049. [DOI] [PubMed] [Google Scholar]
- Pereira M. A., Khoury M. D. Prevention by chemopreventive agents of azoxymethane-induced foci of aberrant crypts in rat colon. Cancer Lett. 1991 Dec 9;61(1):27–33. doi: 10.1016/0304-3835(91)90073-q. [DOI] [PubMed] [Google Scholar]
- Rao C. V., Rivenson A., Simi B., Zang E., Kelloff G., Steele V., Reddy B. S. Chemoprevention of colon carcinogenesis by sulindac, a nonsteroidal anti-inflammatory agent. Cancer Res. 1995 Apr 1;55(7):1464–1472. [PubMed] [Google Scholar]
- Reddy B. S., Maruyama H., Kelloff G. Dose-related inhibition of colon carcinogenesis by dietary piroxicam, a nonsteroidal antiinflammatory drug, during different stages of rat colon tumor development. Cancer Res. 1987 Oct 15;47(20):5340–5346. [PubMed] [Google Scholar]
- Reddy B. S., Rao C. V., Rivenson A., Kelloff G. Inhibitory effect of aspirin on azoxymethane-induced colon carcinogenesis in F344 rats. Carcinogenesis. 1993 Aug;14(8):1493–1497. doi: 10.1093/carcin/14.8.1493. [DOI] [PubMed] [Google Scholar]
- Reddy B. S., Rao C. V., Seibert K. Evaluation of cyclooxygenase-2 inhibitor for potential chemopreventive properties in colon carcinogenesis. Cancer Res. 1996 Oct 15;56(20):4566–4569. [PubMed] [Google Scholar]
- Rubinfeld B., Albert I., Porfiri E., Fiol C., Munemitsu S., Polakis P. Binding of GSK3beta to the APC-beta-catenin complex and regulation of complex assembly. Science. 1996 May 17;272(5264):1023–1026. doi: 10.1126/science.272.5264.1023. [DOI] [PubMed] [Google Scholar]
- Sandler R. S., Galanko J. C., Murray S. C., Helm J. F., Woosley J. T. Aspirin and nonsteroidal anti-inflammatory agents and risk for colorectal adenomas. Gastroenterology. 1998 Mar;114(3):441–447. doi: 10.1016/s0016-5085(98)70526-8. [DOI] [PubMed] [Google Scholar]
- Sano H., Kawahito Y., Wilder R. L., Hashiramoto A., Mukai S., Asai K., Kimura S., Kato H., Kondo M., Hla T. Expression of cyclooxygenase-1 and -2 in human colorectal cancer. Cancer Res. 1995 Sep 1;55(17):3785–3789. [PubMed] [Google Scholar]
- Schnitzler M., Dwight T., Robinson B. G. Sulindac increases the expression of APC mRNA in malignant colonic epithelial cells: an in vitro study. Gut. 1996 May;38(5):707–713. doi: 10.1136/gut.38.5.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwenger P., Bellosta P., Vietor I., Basilico C., Skolnik E. Y., Vilcek J. Sodium salicylate induces apoptosis via p38 mitogen-activated protein kinase but inhibits tumor necrosis factor-induced c-Jun N-terminal kinase/stress-activated protein kinase activation. Proc Natl Acad Sci U S A. 1997 Apr 1;94(7):2869–2873. doi: 10.1073/pnas.94.7.2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiff S. J., Rigas B. Nonsteroidal anti-inflammatory drugs and colorectal cancer: evolving concepts of their chemopreventive actions. Gastroenterology. 1997 Dec;113(6):1992–1998. doi: 10.1016/s0016-5085(97)99999-6. [DOI] [PubMed] [Google Scholar]
- Shivapurkar N., Belinsky S. A., Wolf D. C., Tang Z., Alabaster O. Absence of p53 gene mutations in rat colon carcinomas induced by azoxymethane. Cancer Lett. 1995 Sep 4;96(1):63–70. doi: 10.1016/0304-3835(95)03947-u. [DOI] [PubMed] [Google Scholar]
- Takahashi M., Fukutake M., Yokota S., Ishida K., Wakabayashi K., Sugimura T. Suppression of azoxymethane-induced aberrant crypt foci in rat colon by nimesulide, a selective inhibitor of cyclooxygenase 2. J Cancer Res Clin Oncol. 1996;122(4):219–222. doi: 10.1007/BF01209649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thun M. J., Namboodiri M. M., Heath C. W., Jr Aspirin use and reduced risk of fatal colon cancer. N Engl J Med. 1991 Dec 5;325(23):1593–1596. doi: 10.1056/NEJM199112053252301. [DOI] [PubMed] [Google Scholar]
- Tsujii M., DuBois R. N. Alterations in cellular adhesion and apoptosis in epithelial cells overexpressing prostaglandin endoperoxide synthase 2. Cell. 1995 Nov 3;83(3):493–501. doi: 10.1016/0092-8674(95)90127-2. [DOI] [PubMed] [Google Scholar]
- Valizadeh A., Karayiannakis A. J., el-Hariry I., Kmiot W., Pignatelli M. Expression of E-cadherin-associated molecules (alpha-, beta-, and gamma-catenins and p120) in colorectal polyps. Am J Pathol. 1997 Jun;150(6):1977–1984. [PMC free article] [PubMed] [Google Scholar]
- Vane J. R. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol. 1971 Jun 23;231(25):232–235. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]
- Wallace J. L., Keenan C. M., Granger D. N. Gastric ulceration induced by nonsteroidal anti-inflammatory drugs is a neutrophil-dependent process. Am J Physiol. 1990 Sep;259(3 Pt 1):G462–G467. doi: 10.1152/ajpgi.1990.259.3.G462. [DOI] [PubMed] [Google Scholar]