Abstract
BACKGROUND AND AIMS—Trefoil factor family (TFF) peptides and the chromosome 11p15.5 mucin glycoproteins are expressed and secreted in a site specific fashion along the length of the gastrointestinal tract. Evidence for coexpression of mucins and trefoil peptides has been suggested in numerous gastrointestinal mucosal pathologies. The ulcer associated cell lineage (UACL) occurs at sites of chronic ulceration in Crohn's disease, expresses all three trefoil peptides, and is implicated in mucosal restitution. We tested the hypothesis that individual trefoil peptides are uniquely localised with specific mucins in the UACL and normal gastrointestinal epithelia. METHODS—Expression of mucin genes in the UACL from small bowel tissue of patients with Crohn's disease was detected by in situ hybridisation, and localisation of the products by immunohistochemistry. Colocalisation of mucins and trefoil peptides was demonstrated by immunofluorescent colabelling in UACL and normal gastrointestinal epithelia. RESULTS—MUC5AC and TFF1 were colocalised in distal ductular and surface elements of the UACL and in foveolar cells of the stomach, whereas MUC6 and TFF2 were colocalised to acinar and proximal ductular structures in the UACL, in the fundus and deep antral glands of the stomach, and in Brunner's glands of the duodenum. MUC5B was found sporadically throughout the UACL and gastric body. MUC2 was absent from the UACL, Brunner's glands, and stomach. MUC2 and TFF3 were colocalised throughout the large and small bowel mucosa. CONCLUSIONS—The UACL has a unique profile of mucin gene expression. Coordinated localisation of trefoil peptides and mucins in UACL and normal gastrointestinal epithelia suggests they may assist each others' functions in protection and repair of gastrointestinal mucosa. Keywords: mucins; trefoil peptides; Crohn's disease; ulcer associated cell lineage; repair
Full Text
The Full Text of this article is available as a PDF (371.8 KB).
Figure 1 .

Sections of terminal ileum from a patient with active Crohn's disease showing tissue morphology and mucin gene expression. (A) Haematoxylin and eosin stain, with ulcer associated cell lineage (UACL) glands indicated by an arrow. (B) Periodic acid Schiff/alcian blue stained goblet cell mucins blue/purple and UACL glands magenta. (C) MUC2 glycoprotein immunohistochemical localisation (brown) confined to goblet cells, corresponding to the pattern of expression of (D) MUC2 mRNA. (E, F) MUC5AC glycoprotein and mRNA localised to some surface and upper ductular compartments of UACL glands. (G, H) MUC5B glycoprotein and mRNA localised to some surface and mid deep compartments of the UACL glands. (I, J) MUC6 glycoprotein and mRNA are abundant in the acinar and ductular compartments of the UCAL, with some MUC6 glycoprotein also in the surface cells. All immunohistochemical sections were counterstained with haematoxylin, and all in situ hybridisation sections counterstained with toluidine blue. Original magnifications: A-J 50×.
Figure 2 .
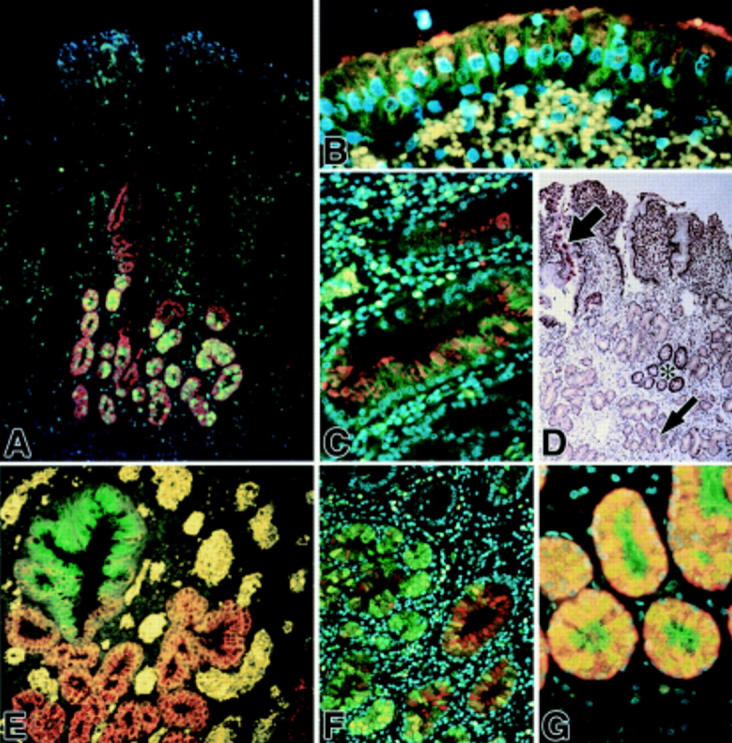
Fluorescent immunohistochemical localisation of mucin glycoproteins and trefoil factor family (TFF) peptides in ulcer associated cell lineage (UACL) glands. (A) TFF2 (green) appears to be confined to the acinar and lower ductular compartments; MUC6 (red) is seen throughout the acini and some ductular components; colocalisation is seen as orange-yellow in many acinar cells. Nuclei are counterstained blue with DAPI. (B, C) TFF1 (green) and MUC5AC (red) are located in UACL surface cells (B) and ducts (C); colocalisation is frequent and seen as orange-yellow. Erythrocytes in the lamina propria autofluoresce an intense yellow (DAPI counterstain). (D) Conventional immunohistochemical localisation of TFF3 (brown) reveals strong staining in crypts (asterisk) and also in UACL (small arrow), particularly in the surface cells (large arrow) (haematoxylin counterstain). (E) TFF1 (green) and MUC6 (red) are present in opposing gradients; TFF1 is localised most strongly in the surface cells and upper ducts whereas MUC6 alone is present in acinar cells (red; bottom and right) and colocalisation (orange-yellow) is seen through the ducts. Erythrocytes in the lamina propria autofluoresce an intense yellow (weak DAPI counterstain). (F) TFF2 (green) and MUC5B (red) colocalise in some acinar and duct cells (DAPI counterstain). (G) TFF2 (green) and MUC6 (red) are both abundant in acinar cells of the UACL (DAPI counterstain). Original magnifications: A 100×; B, C, and G 400×; D 50×; E and F 200×.
Figure 3 .
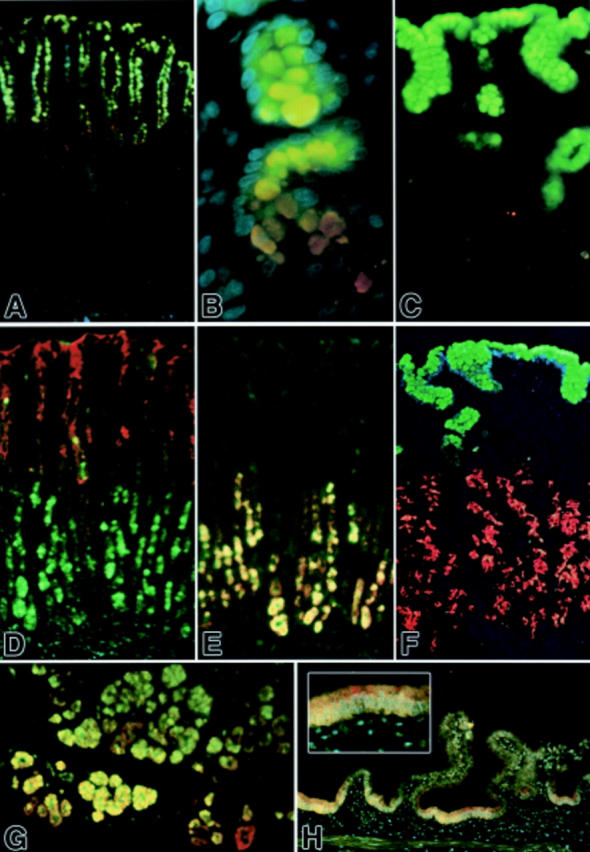
Fluorescent immunohistochemical localisation of mucin glycoproteins and trefoil factor family (TFF) peptides in the stomach, duodenum, and gall bladder. (A, B) TFF1 (green) and MUC5AC (red) colocalised in the gastric surface epithelium; MUC5AC immunoreactivity continues deeper into the isthmus and neck region (DAPI counterstain). (C) TFF1 (green) appears abundant in gastric foveolar surface cells whereas MUC5B (red) appears weakly localised to some mucous neck cells (weak DAPI counterstain). (D) TFF2 (green) and MUC5AC (red) were mostly segregated, with TFF2 immunoreactivity abundant in basal parts of these gastric glands and MUC5AC glycoprotein abundant in the epithelium at the surface, isthmus, and neck. Colocalisation was infrequent (DAPI counterstain). (E) TFF2 (green) and MUC6 glycoprotein (red) were mostly colocalised and confined to the basal parts of these gastric glands. (F) TFF1 (green) and MUC6 glycoprotein (red) were mostly segregated, with TFF1 abundant in surface epithelium and MUC6 appearing confined to epithelium in the lower half of these gastric glands, including some unequivocal mucous neck cells (red; upper left) (DAPI counterstain). (G) TFF2 (green) and MUC6 glycoprotein (red) were abundant in epithelium in the acini of Brunner's glands of the duodenum, and are most often colocalised (weak DAPI counterstain). (H) TFF2 (green) and MUC6 glycoprotein (red) appear colocalised (orange-yellow) in some epithelium of this non-inflamed gall bladder. Inset shows enlargement of central region (DAPI counterstain). Original magnifications: A, D, E, H 100×; B 400×; C 250×; F 200×.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahnen D. J., Poulsom R., Stamp G. W., Elia G., Pike C., Jeffery R., Longcroft J., Rio M. C., Chambon P., Wright N. A. The ulceration-associated cell lineage (UACL) reiterates the Brunner's gland differentiation programme but acquires the proliferative organization of the gastric gland. J Pathol. 1994 Aug;173(4):317–326. doi: 10.1002/path.1711730406. [DOI] [PubMed] [Google Scholar]
- Audie J. P., Janin A., Porchet N., Copin M. C., Gosselin B., Aubert J. P. Expression of human mucin genes in respiratory, digestive, and reproductive tracts ascertained by in situ hybridization. J Histochem Cytochem. 1993 Oct;41(10):1479–1485. doi: 10.1177/41.10.8245407. [DOI] [PubMed] [Google Scholar]
- Bobek L. A., Tsai H., Biesbrock A. R., Levine M. J. Molecular cloning, sequence, and specificity of expression of the gene encoding the low molecular weight human salivary mucin (MUC7). J Biol Chem. 1993 Sep 25;268(27):20563–20569. [PubMed] [Google Scholar]
- Buisine M. P., Janin A., Maunoury V., Audié J. P., Delescaut M. P., Copin M. C., Colombel J. F., Degand P., Aubert J. P., Porchet N. Aberrant expression of a human mucin gene (MUC5AC) in rectosigmoid villous adenoma. Gastroenterology. 1996 Jan;110(1):84–91. doi: 10.1053/gast.1996.v110.pm8536891. [DOI] [PubMed] [Google Scholar]
- De Bolós C., Garrido M., Real F. X. MUC6 apomucin shows a distinct normal tissue distribution that correlates with Lewis antigen expression in the human stomach. Gastroenterology. 1995 Sep;109(3):723–734. doi: 10.1016/0016-5085(95)90379-8. [DOI] [PubMed] [Google Scholar]
- Dignass A., Lynch-Devaney K., Kindon H., Thim L., Podolsky D. K. Trefoil peptides promote epithelial migration through a transforming growth factor beta-independent pathway. J Clin Invest. 1994 Jul;94(1):376–383. doi: 10.1172/JCI117332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufosse J., Porchet N., Audie J. P., Guyonnet Duperat V., Laine A., Van-Seuningen I., Marrakchi S., Degand P., Aubert J. P. Degenerate 87-base-pair tandem repeats create hydrophilic/hydrophobic alternating domains in human mucin peptides mapped to 11p15. Biochem J. 1993 Jul 15;293(Pt 2):329–337. doi: 10.1042/bj2930329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efstathiou J. A., Noda M., Rowan A., Dixon C., Chinery R., Jawhari A., Hattori T., Wright N. A., Bodmer W. F., Pignatelli M. Intestinal trefoil factor controls the expression of the adenomatous polyposis coli-catenin and the E-cadherin-catenin complexes in human colon carcinoma cells. Proc Natl Acad Sci U S A. 1998 Mar 17;95(6):3122–3127. doi: 10.1073/pnas.95.6.3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendler S. J., Lancaster C. A., Taylor-Papadimitriou J., Duhig T., Peat N., Burchell J., Pemberton L., Lalani E. N., Wilson D. Molecular cloning and expression of human tumor-associated polymorphic epithelial mucin. J Biol Chem. 1990 Sep 5;265(25):15286–15293. [PubMed] [Google Scholar]
- Gum J. R., Hicks J. W., Swallow D. M., Lagace R. L., Byrd J. C., Lamport D. T., Siddiki B., Kim Y. S. Molecular cloning of cDNAs derived from a novel human intestinal mucin gene. Biochem Biophys Res Commun. 1990 Aug 31;171(1):407–415. doi: 10.1016/0006-291x(90)91408-k. [DOI] [PubMed] [Google Scholar]
- Gum J. R., Jr, Hicks J. W., Toribara N. W., Siddiki B., Kim Y. S. Molecular cloning of human intestinal mucin (MUC2) cDNA. Identification of the amino terminus and overall sequence similarity to prepro-von Willebrand factor. J Biol Chem. 1994 Jan 28;269(4):2440–2446. [PubMed] [Google Scholar]
- Guyonnet Duperat V., Audie J. P., Debailleul V., Laine A., Buisine M. P., Galiegue-Zouitina S., Pigny P., Degand P., Aubert J. P., Porchet N. Characterization of the human mucin gene MUC5AC: a consensus cysteine-rich domain for 11p15 mucin genes? Biochem J. 1995 Jan 1;305(Pt 1):211–219. doi: 10.1042/bj3050211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanby A. M., Poulsom R., Elia G., Singh S., Longcroft J. M., Wright N. A. The expression of the trefoil peptides pS2 and human spasmolytic polypeptide (hSP) in 'gastric metaplasia' of the proximal duodenum: implications for the nature of 'gastric metaplasia'. J Pathol. 1993 Mar;169(3):355–360. doi: 10.1002/path.1711690313. [DOI] [PubMed] [Google Scholar]
- Hanby A. M., Poulsom R., Singh S., Elia G., Jeffery R. E., Wright N. A. Spasmolytic polypeptide is a major antral peptide: distribution of the trefoil peptides human spasmolytic polypeptide and pS2 in the stomach. Gastroenterology. 1993 Oct;105(4):1110–1116. doi: 10.1016/0016-5085(93)90956-d. [DOI] [PubMed] [Google Scholar]
- Hauser F., Poulsom R., Chinery R., Rogers L. A., Hanby A. M., Wright N. A., Hoffmann W. hP1.B, a human P-domain peptide homologous with rat intestinal trefoil factor, is expressed also in the ulcer-associated cell lineage and the uterus. Proc Natl Acad Sci U S A. 1993 Aug 1;90(15):6961–6965. doi: 10.1073/pnas.90.15.6961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry J. A., Bennett M. K., Piggott N. H., Levett D. L., May F. E., Westley B. R. Expression of the pNR-2/pS2 protein in diverse human epithelial tumours. Br J Cancer. 1991 Oct;64(4):677–682. doi: 10.1038/bjc.1991.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho S. B., Roberton A. M., Shekels L. L., Lyftogt C. T., Niehans G. A., Toribara N. W. Expression cloning of gastric mucin complementary DNA and localization of mucin gene expression. Gastroenterology. 1995 Sep;109(3):735–747. doi: 10.1016/0016-5085(95)90380-1. [DOI] [PubMed] [Google Scholar]
- Hovenberg H. W., Davies J. R., Carlstedt I. Different mucins are produced by the surface epithelium and the submucosa in human trachea: identification of MUC5AC as a major mucin from the goblet cells. Biochem J. 1996 Aug 15;318(Pt 1):319–324. doi: 10.1042/bj3180319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovenberg H. W., Davies J. R., Herrmann A., Lindén C. J., Carlstedt I. MUC5AC, but not MUC2, is a prominent mucin in respiratory secretions. Glycoconj J. 1996 Oct;13(5):839–847. doi: 10.1007/BF00702348. [DOI] [PubMed] [Google Scholar]
- Kindon H., Pothoulakis C., Thim L., Lynch-Devaney K., Podolsky D. K. Trefoil peptide protection of intestinal epithelial barrier function: cooperative interaction with mucin glycoprotein. Gastroenterology. 1995 Aug;109(2):516–523. doi: 10.1016/0016-5085(95)90340-2. [DOI] [PubMed] [Google Scholar]
- Klomp L. W., Van Rens L., Strous G. J. Cloning and analysis of human gastric mucin cDNA reveals two types of conserved cysteine-rich domains. Biochem J. 1995 Jun 15;308(Pt 3):831–838. doi: 10.1042/bj3080831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klomp L. W., van Rens L., Strous G. J. Identification of a human gastric mucin precursor: N-linked glycosylation and oligomerization. Biochem J. 1994 Dec 15;304(Pt 3):693–698. doi: 10.1042/bj3040693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEE F. D. PYLORIC METAPLASIA IN THE SMALL INTESTINE. J Pathol Bacteriol. 1964 Apr;87:267–277. doi: 10.1002/path.1700870207. [DOI] [PubMed] [Google Scholar]
- LIBER A. F. Aberrant pyloric glands in regional ileitis. AMA Arch Pathol. 1951 Feb;51(2):205–212. [PubMed] [Google Scholar]
- Labouvie C., Machado J. C., Carneiro F., Sarbia M., Vieth M., Porschen R., Seitz G., Blin N. Differential expression of mucins and trefoil peptides in native epithelium, Barrett's metaplasia and squamous cell carcinoma of the oesophagus. J Cancer Res Clin Oncol. 1999;125(2):71–76. doi: 10.1007/s004320050244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meezaman D., Charles P., Daskal E., Polymeropoulos M. H., Martin B. M., Rose M. C. Cloning and analysis of cDNA encoding a major airway glycoprotein, human tracheobronchial mucin (MUC5). J Biol Chem. 1994 Apr 29;269(17):12932–12939. [PubMed] [Google Scholar]
- Patel K., Hanby A. M., Ahnen D. J., Playford R. J., Wright N. A. The kinetic organization of the ulcer-associated cell lineage (UACL): delineation of a novel putative stem-cell region. Epithelial Cell Biol. 1994;3(4):156–160. [PubMed] [Google Scholar]
- Pigny P., Guyonnet-Duperat V., Hill A. S., Pratt W. S., Galiegue-Zouitina S., d'Hooge M. C., Laine A., Van-Seuningen I., Degand P., Gum J. R. Human mucin genes assigned to 11p15.5: identification and organization of a cluster of genes. Genomics. 1996 Dec 15;38(3):340–352. doi: 10.1006/geno.1996.0637. [DOI] [PubMed] [Google Scholar]
- Podolsky D. K., Lynch-Devaney K., Stow J. L., Oates P., Murgue B., DeBeaumont M., Sands B. E., Mahida Y. R. Identification of human intestinal trefoil factor. Goblet cell-specific expression of a peptide targeted for apical secretion. J Biol Chem. 1993 Mar 25;268(9):6694–6702. [PubMed] [Google Scholar]
- Podolsky D. K. The colonic goblet cell and glycoprotein heterogeneity. Immunol Invest. 1989 Jan-May;18(1-4):485–497. doi: 10.3109/08820138909112258. [DOI] [PubMed] [Google Scholar]
- Porchet N., Nguyen V. C., Dufosse J., Audie J. P., Guyonnet-Duperat V., Gross M. S., Denis C., Degand P., Bernheim A., Aubert J. P. Molecular cloning and chromosomal localization of a novel human tracheo-bronchial mucin cDNA containing tandemly repeated sequences of 48 base pairs. Biochem Biophys Res Commun. 1991 Mar 15;175(2):414–422. doi: 10.1016/0006-291x(91)91580-6. [DOI] [PubMed] [Google Scholar]
- Poulsom R., Chinery R., Sarraf C., Van Noorden S., Stamp G. W., Lalani E. N., Elia G., Wright N. A. Trefoil peptide gene expression in small intestinal Crohn's disease and dietary adaptation. J Clin Gastroenterol. 1993;17 (Suppl 1):S78–S91. doi: 10.1097/00004836-199312001-00016. [DOI] [PubMed] [Google Scholar]
- Rio M. C., Bellocq J. P., Daniel J. Y., Tomasetto C., Lathe R., Chenard M. P., Batzenschlager A., Chambon P. Breast cancer-associated pS2 protein: synthesis and secretion by normal stomach mucosa. Science. 1988 Aug 5;241(4866):705–708. doi: 10.1126/science.3041593. [DOI] [PubMed] [Google Scholar]
- Rio M. C., Chenard M. P., Wolf C., Marcellin L., Tomasetto C., Lathe R., Bellocq J. P., Chambon P. Induction of pS2 and hSP genes as markers of mucosal ulceration of the digestive tract. Gastroenterology. 1991 Feb;100(2):375–379. doi: 10.1016/0016-5085(91)90205-y. [DOI] [PubMed] [Google Scholar]
- Roberts I. S., Stoddart R. W. Ulcer-associated cell lineage ('pyloric metaplasia') in Crohn's disease: a lectin histochemical study. J Pathol. 1993 Sep;171(1):13–19. doi: 10.1002/path.1711710105. [DOI] [PubMed] [Google Scholar]
- Schmidt P. H., Lee J. R., Joshi V., Playford R. J., Poulsom R., Wright N. A., Goldenring J. R. Identification of a metaplastic cell lineage associated with human gastric adenocarcinoma. Lab Invest. 1999 Jun;79(6):639–646. [PMC free article] [PubMed] [Google Scholar]
- Seib T., Blin N., Hilgert K., Seifert M., Theisinger B., Engel M., Dooley S., Zang K. D., Welter C. The three human trefoil genes TFF1, TFF2, and TFF3 are located within a region of 55 kb on chromosome 21q22.3. Genomics. 1997 Feb 15;40(1):200–202. doi: 10.1006/geno.1996.4511. [DOI] [PubMed] [Google Scholar]
- Shankar V., Gilmore M. S., Elkins R. C., Sachdev G. P. A novel human airway mucin cDNA encodes a protein with unique tandem-repeat organization. Biochem J. 1994 Jun 1;300(Pt 2):295–298. doi: 10.1042/bj3000295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suemori S., Lynch-Devaney K., Podolsky D. K. Identification and characterization of rat intestinal trefoil factor: tissue- and cell-specific member of the trefoil protein family. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11017–11021. doi: 10.1073/pnas.88.24.11017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton D. J., Howard M., Khan N., Sheehan J. K. Identification of two glycoforms of the MUC5B mucin in human respiratory mucus. Evidence for a cysteine-rich sequence repeated within the molecule. J Biol Chem. 1997 Apr 4;272(14):9561–9566. doi: 10.1074/jbc.272.14.9561. [DOI] [PubMed] [Google Scholar]
- Tomasetto C., Masson R., Linares J. L., Wendling C., Lefebvre O., Chenard M. P., Rio M. C. pS2/TFF1 interacts directly with the VWFC cysteine-rich domains of mucins. Gastroenterology. 2000 Jan;118(1):70–80. doi: 10.1016/s0016-5085(00)70415-x. [DOI] [PubMed] [Google Scholar]
- Tomasetto C., Rio M. C., Gautier C., Wolf C., Hareuveni M., Chambon P., Lathe R. hSP, the domain-duplicated homolog of pS2 protein, is co-expressed with pS2 in stomach but not in breast carcinoma. EMBO J. 1990 Feb;9(2):407–414. doi: 10.1002/j.1460-2075.1990.tb08125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toribara N. W., Roberton A. M., Ho S. B., Kuo W. L., Gum E., Hicks J. W., Gum J. R., Jr, Byrd J. C., Siddiki B., Kim Y. S. Human gastric mucin. Identification of a unique species by expression cloning. J Biol Chem. 1993 Mar 15;268(8):5879–5885. [PubMed] [Google Scholar]
- Tytgat K. M., Büller H. A., Opdam F. J., Kim Y. S., Einerhand A. W., Dekker J. Biosynthesis of human colonic mucin: Muc2 is the prominent secretory mucin. Gastroenterology. 1994 Nov;107(5):1352–1363. doi: 10.1016/0016-5085(94)90537-1. [DOI] [PubMed] [Google Scholar]
- Van Klinken B. J., Dekker J., Büller H. A., de Bolòs C., Einerhand A. W. Biosynthesis of mucins (MUC2-6) along the longitudinal axis of the human gastrointestinal tract. Am J Physiol. 1997 Aug;273(2 Pt 1):G296–G302. doi: 10.1152/ajpgi.1997.273.2.G296. [DOI] [PubMed] [Google Scholar]
- Weiss A. A., Babyatsky M. W., Ogata S., Chen A., Itzkowitz S. H. Expression of MUC2 and MUC3 mRNA in human normal, malignant, and inflammatory intestinal tissues. J Histochem Cytochem. 1996 Oct;44(10):1161–1166. doi: 10.1177/44.10.8813081. [DOI] [PubMed] [Google Scholar]
- Williams S. J., McGuckin M. A., Gotley D. C., Eyre H. J., Sutherland G. R., Antalis T. M. Two novel mucin genes down-regulated in colorectal cancer identified by differential display. Cancer Res. 1999 Aug 15;59(16):4083–4089. [PubMed] [Google Scholar]
- Wright N. A., Pike C., Elia G. Induction of a novel epidermal growth factor-secreting cell lineage by mucosal ulceration in human gastrointestinal stem cells. Nature. 1990 Jan 4;343(6253):82–85. doi: 10.1038/343082a0. [DOI] [PubMed] [Google Scholar]
- Wright N. A., Poulsom R., Stamp G. W., Hall P. A., Jeffery R. E., Longcroft J. M., Rio M. C., Tomasetto C., Chambon P. Epidermal growth factor (EGF/URO) induces expression of regulatory peptides in damaged human gastrointestinal tissues. J Pathol. 1990 Dec;162(4):279–284. doi: 10.1002/path.1711620402. [DOI] [PubMed] [Google Scholar]
- Wright N. A., Poulsom R., Stamp G., Van Noorden S., Sarraf C., Elia G., Ahnen D., Jeffery R., Longcroft J., Pike C. Trefoil peptide gene expression in gastrointestinal epithelial cells in inflammatory bowel disease. Gastroenterology. 1993 Jan;104(1):12–20. doi: 10.1016/0016-5085(93)90830-6. [DOI] [PubMed] [Google Scholar]
- van Klinken B. J., Dekker J., van Gool S. A., van Marle J., Büller H. A., Einerhand A. W. MUC5B is the prominent mucin in human gallbladder and is also expressed in a subset of colonic goblet cells. Am J Physiol. 1998 May;274(5 Pt 1):G871–G878. doi: 10.1152/ajpgi.1998.274.5.G871. [DOI] [PubMed] [Google Scholar]
- van Klinken B. J., Dekker J., van Gool S. A., van Marle J., Büller H. A., Einerhand A. W. MUC5B is the prominent mucin in human gallbladder and is also expressed in a subset of colonic goblet cells. Am J Physiol. 1998 May;274(5 Pt 1):G871–G878. doi: 10.1152/ajpgi.1998.274.5.G871. [DOI] [PubMed] [Google Scholar]


