Abstract
BACKGROUND—Lung injury manifest clinically as adult respiratory distress syndrome (ARDS) is a common cause of morbidity and mortality following acute pancreatitis (AP). Neutrophils play a critical role in the progression of AP to ARDS. C-x-C chemokines are potent neutrophil chemoattractants and activators and have been implicated in AP. AIMS—To evaluate the effect of blocking the C-x-C chemokine, cytokine induced neutrophil chemoattractant (CINC), in AP on pancreatic inflammation and the associated lung injury in rats. METHODS—AP was induced by hourly intraperitoneal injections of caerulein. Goat anti-CINC antibody was administered either before or after starting caerulein injections to evaluate the prophylactic and therapeutic effects, respectively. Severity of AP was determined by measuring plasma amylase, pancreatic water content, and pancreatic myeloperoxidase (MPO) activity as a measure of neutrophil sequestration in the pancreas. Lung injury was determined by measurement of pulmonary microvascular permeability and lung MPO activity. RESULTS—Treatment with anti-CINC antibody had little effect on caerulein induced pancreatic damage. However, it reduced the caerulein mediated increase in lung MPO activity as well as lung microvascular permeability when administered either prophylactically (lung MPO (fold increase over control): 1.53 (0.21) v 3.30 (0.46), p<0.05; microvascular permeability (L/P%): 0.42 (0.07) v 0.77 (0.11), p<0.05) or therapeutically (lung MPO (fold increase over control): 2.13 (0.10) v 4.42 (0.65), p<0.05; microvascular permeability (L/P%): 0.31 (0.05) v 0.79 (0.13), p<0.05). CONCLUSION—Treatment with anti-CINC antibody afforded significant protection against pancreatitis associated lung injury. These results suggest that CINC plays an important role in the systemic inflammatory response in AP. Keywords: chemokines; acute pancreatitis; caerulein; adult respiratory distress syndrome
Full Text
The Full Text of this article is available as a PDF (266.7 KB).
Figure 1 .
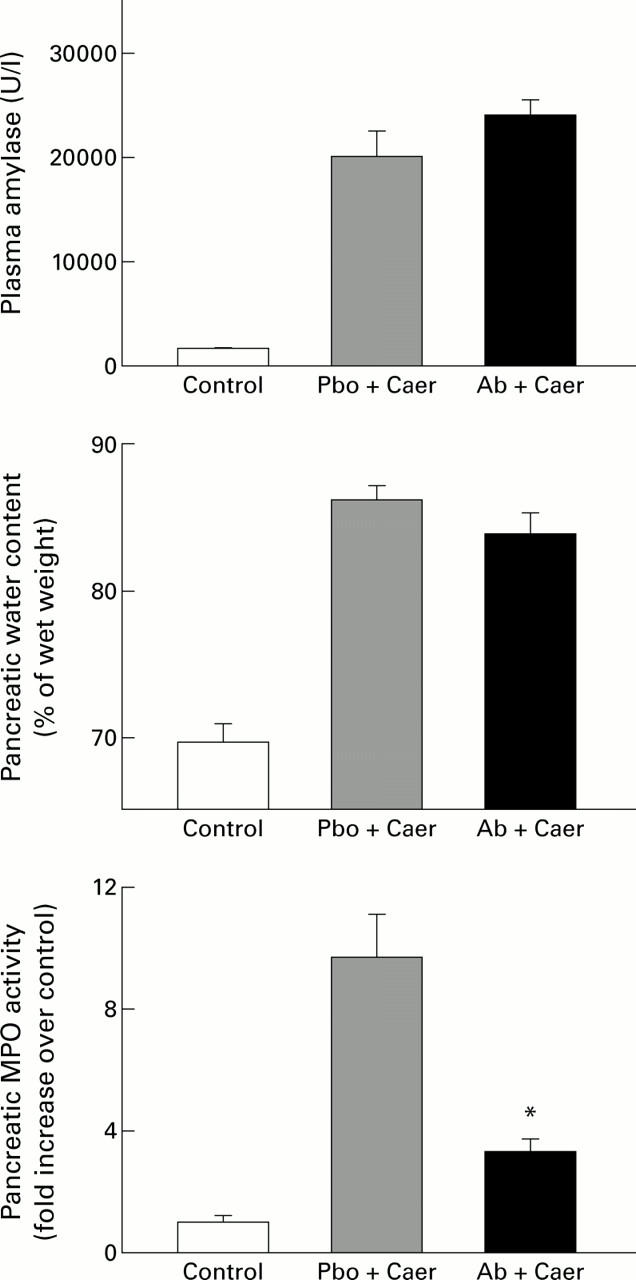
Effects of prophylactic treatment with anti-cytokine induced neutrophil chemoattractant antibody (anti-CINC antibody) on acute pancreatitis. The results shown are mean (SEM) for eight animals in each group. The results are as follows: plasma amylase (U/l)—control 1593 (54); placebo (Pbo) followed by caerulein (Caer) 20 206 (2374); anti-CINC antibody (Ab) followed by Caer 24 266 (1391); pancreatic water content (% of wet weight)—control 69.7 (1.2); Pbo followed by Caer 86.4 (1.0); Ab followed by Caer 84.1 (1.4); pancreatic myeloperoxidase (MPO) activity (fold increase over control)—control 1.0 (0.26); Pbo followed by Caer 9.72 (1.43); Ab followed by Caer 3.67 (0.38). *p<0.05, antibody treated animals with acute pancreatitis compared with placebo group.
Figure 2 .
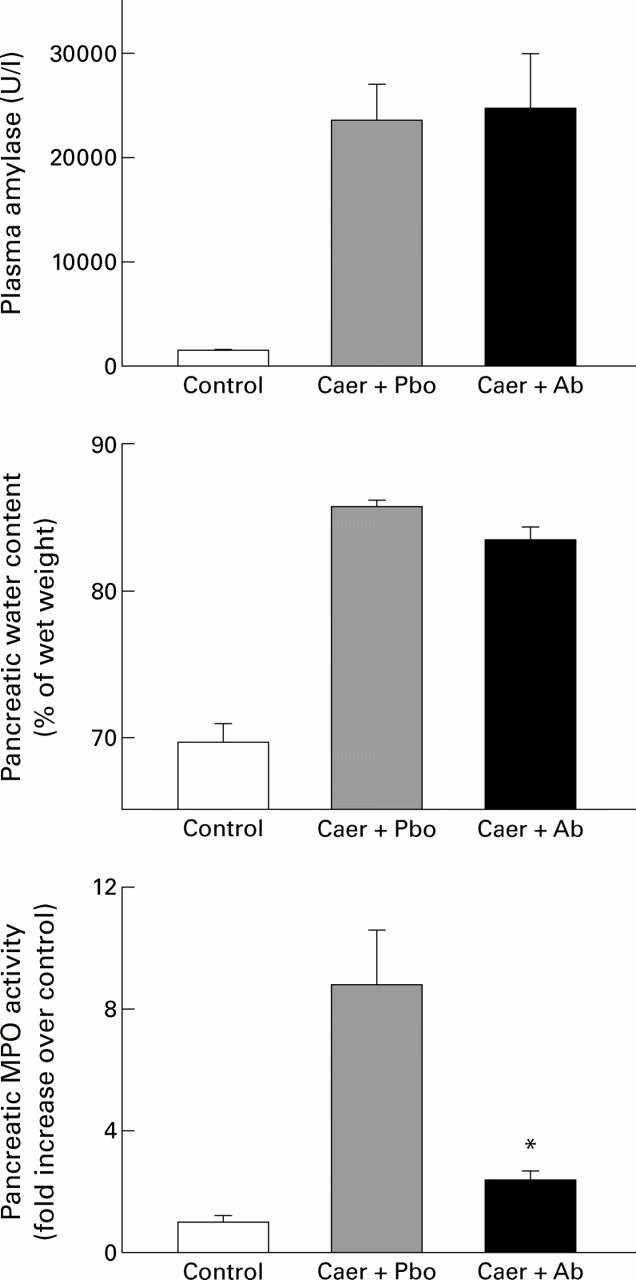
Effects of therapeutic treatment with anti-cytokine induced neutrophil chemoattractant antibody (anti-CINC antibody) on acute pancreatitis. The results shown are the mean (SEM) for eight animals in each group. The results are as follows: plasma amylase (U/l)—control 1593 (54); caerulein (Caer) followed by placebo (Pbo) 23 860 (3377); Caer followed by anti-CINC antibody (Ab) 25 046 (5337); pancreatic water content (% of wet weight)—control 69.7 (1.2); Caer followed by Pbo 86.1 (0.5); Caer followed by Ab 83.8 (0.9); pancreatic myeloperoxidase (MPO) activity (fold increase over control)—control 1.0 (0.26); Caer followed by Pbo 8.86 (1.8); Caer followed by Ab 2.38 (0.3). *p<0.05, antibody treated animals with acute pancreatitis compared with placebo group.
Figure 3 .
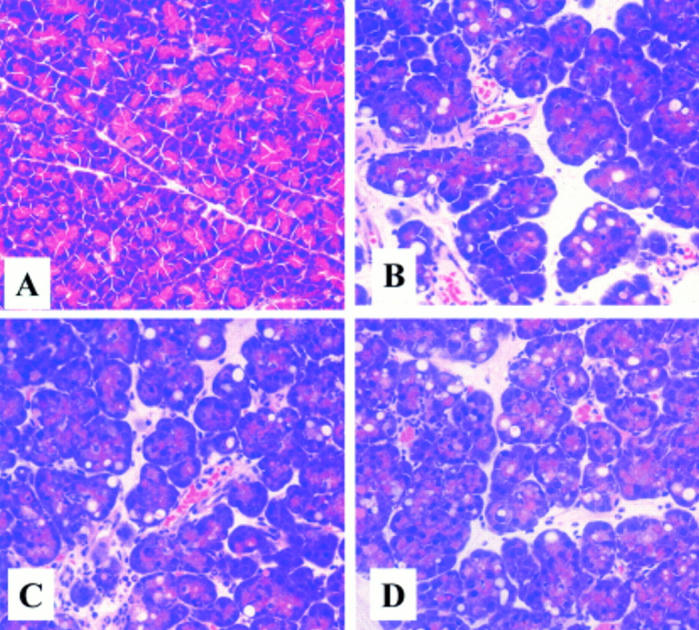
Morphological changes in rat pancreas on induction of acute pancreatitis with/without treatment with anti-cytokine induced neutrophil chemoattractant antibody (anti-CINC antibody). (A) Control: no pancreatitis. (B) Caerulein induced acute pancreatitis with placebo. (C) Caerulein induced acute pancreatitis with prophylactic treatment with anti-CINC antibody. (D) Caerulein induced acute pancreatitis with therapeutic treatment with anti-CINC antibody.
Figure 4 .
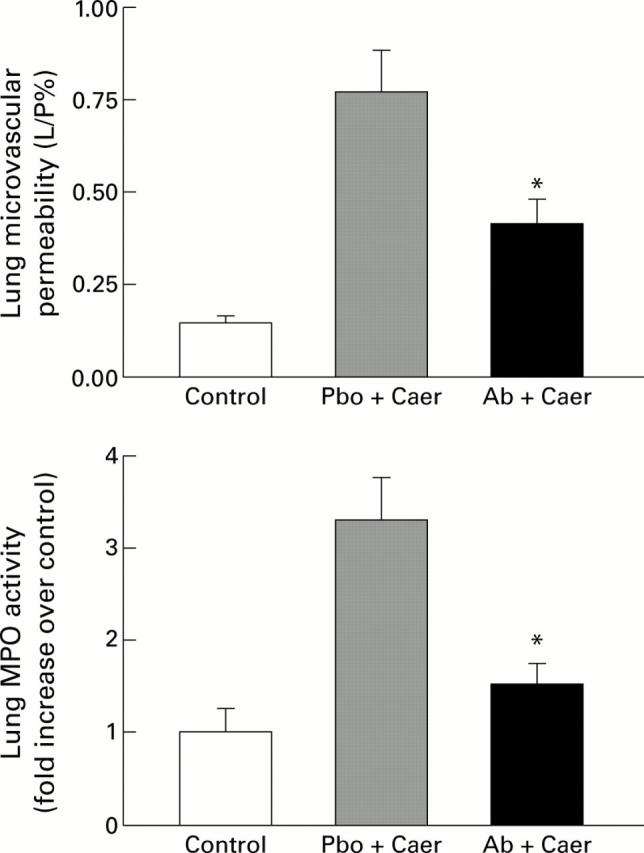
Effects of prophylactic treatment with anti-cytokine induced neutrophil chemoattractant antibody (anti-CINC antibody) on acute pancreatitis associated lung injury. The results shown are mean (SEM) for eight animals in each group. The results are as follows: pulmonary microvascular permeability (L/P%)—control 0.15 (0.02); placebo (Pbo) followed by caerulein (Caer) 0.78 (0.11); anti-CINC antibody (Ab) followed by Caer 0.42 (0.04); lung myeloperoxidase (MPO) activity (fold increase over control)—control 1.0 (0.26); Pbo followed by Caer 3.30 (0.46); Ab followed by Caer 1.53 (0.21). *p<0.05, antibody treated animals with acute pancreatitis compared with placebo group group. L/P, lavage to plasma FITC-albumin fluorescence ratio.
Figure 5 .
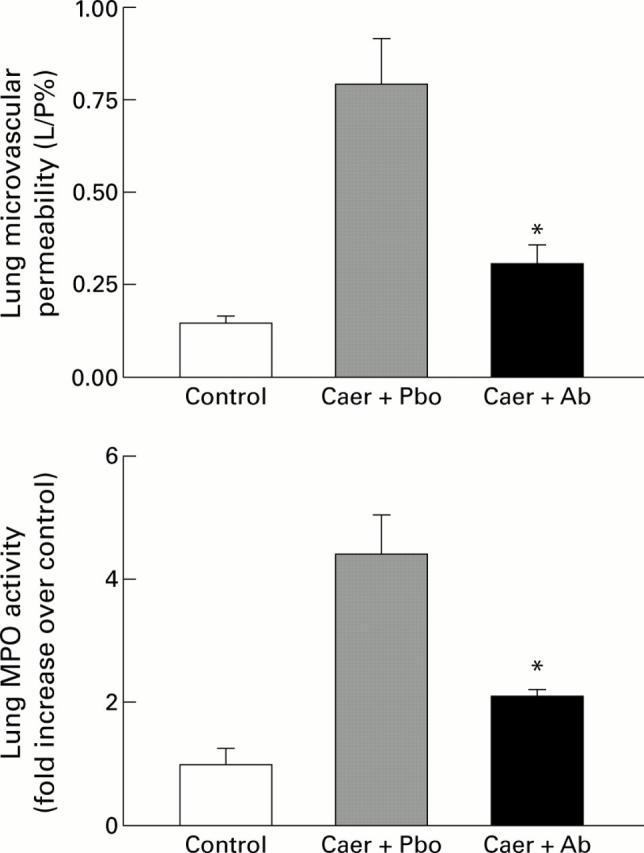
Effects of therapeutic treatment with anti-cytokine induced neutrophil chemoattractant antibody (anti-CINC antibody) on acute pancreatitis associated lung injury. The results shown are mean (SEM) for eight animals in each group. The results are as follows: pulmonary microvascular permeability (L/P%)—control 0.15 (0.02); caerulein (Caer) followed by placebo (Pbo) 0.79 (0.12); Caer followed by anti-CINC antibody (Ab) 0.31 (0.05); lung myeloperoxidase (MPO) activity (fold increase over control)—control 1.0 (0.26); Caer followed by Pbo 4.42 (0.64); Caer followed by Ab 2.13 (0.10). *p<0.05, antibody treated animals with acute pancreatitis compared with placebo group. L/P, lavage to plasma FITC-albumin fluorescence ratio.
Figure 6 .
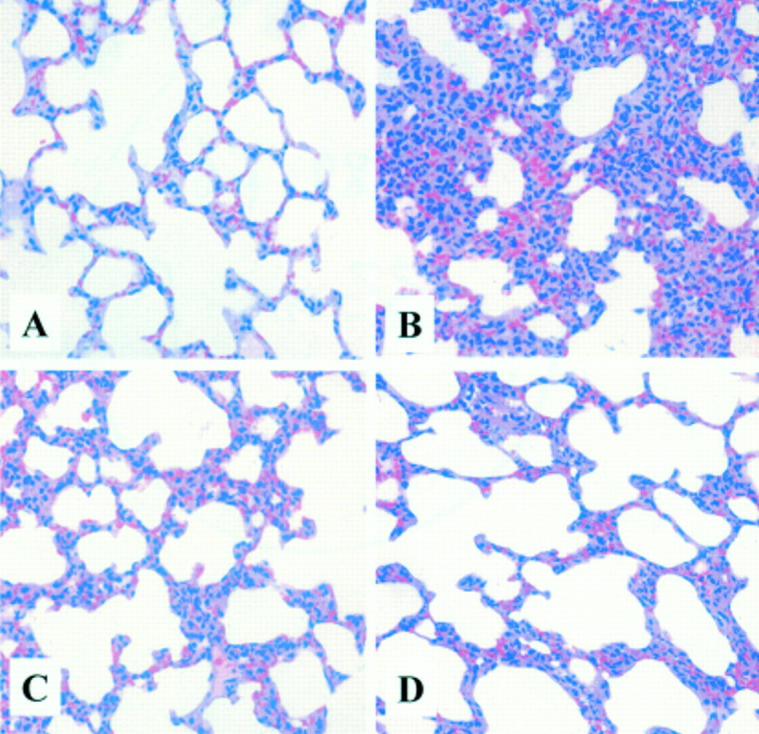
Morphological changes in rat lung on induction of acute pancreatitis with/without treatment with anti-cytokine induced neutrophil chemoattractant antibody (anti-CINC antibody). (A) Control: no pancreatitis. (B) Caerulein induced acute pancreatitis with placebo. (C) Caerulein induced acute pancreatitis with prophylactic treatment with anti-CINC antibody. (D) Caerulein induced acute pancreatitis with therapeutic treatment with anti-CINC antibody.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. H., Lloyd A. R. Chemokines: leucocyte recruitment and activation cytokines. Lancet. 1997 Feb 15;349(9050):490–495. doi: 10.1016/s0140-6736(96)07524-1. [DOI] [PubMed] [Google Scholar]
- Adams D. H., Shaw S. Leucocyte-endothelial interactions and regulation of leucocyte migration. Lancet. 1994 Apr 2;343(8901):831–836. doi: 10.1016/s0140-6736(94)92029-x. [DOI] [PubMed] [Google Scholar]
- Baggiolini M. Chemokines and leukocyte traffic. Nature. 1998 Apr 9;392(6676):565–568. doi: 10.1038/33340. [DOI] [PubMed] [Google Scholar]
- Berney T., Gasche Y., Robert J., Jenny A., Mensi N., Grau G., Vermeulen B., Morel P. Serum profiles of interleukin-6, interleukin-8, and interleukin-10 in patients with severe and mild acute pancreatitis. Pancreas. 1999 May;18(4):371–377. doi: 10.1097/00006676-199905000-00007. [DOI] [PubMed] [Google Scholar]
- Bhatia M., Brady M., Shokuhi S., Christmas S., Neoptolemos J. P., Slavin J. Inflammatory mediators in acute pancreatitis. J Pathol. 2000 Feb;190(2):117–125. doi: 10.1002/(SICI)1096-9896(200002)190:2<117::AID-PATH494>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Bhatia M., Saluja A. K., Hofbauer B., Frossard J. L., Lee H. S., Castagliuolo I., Wang C. C., Gerard N., Pothoulakis C., Steer M. L. Role of substance P and the neurokinin 1 receptor in acute pancreatitis and pancreatitis-associated lung injury. Proc Natl Acad Sci U S A. 1998 Apr 14;95(8):4760–4765. doi: 10.1073/pnas.95.8.4760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatia M., Saluja A. K., Hofbauer B., Lee H. S., Frossard J. L., Steer M. L. The effects of neutrophil depletion on a completely noninvasive model of acute pancreatitis-associated lung injury. Int J Pancreatol. 1998 Oct;24(2):77–83. doi: 10.1007/BF02788564. [DOI] [PubMed] [Google Scholar]
- Butcher E. C. Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell. 1991 Dec 20;67(6):1033–1036. doi: 10.1016/0092-8674(91)90279-8. [DOI] [PubMed] [Google Scholar]
- Butcher E. C., Picker L. J. Lymphocyte homing and homeostasis. Science. 1996 Apr 5;272(5258):60–66. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- Campbell J. J., Hedrick J., Zlotnik A., Siani M. A., Thompson D. A., Butcher E. C. Chemokines and the arrest of lymphocytes rolling under flow conditions. Science. 1998 Jan 16;279(5349):381–384. doi: 10.1126/science.279.5349.381. [DOI] [PubMed] [Google Scholar]
- Colletti L. M., Kunkel S. L., Walz A., Burdick M. D., Kunkel R. G., Wilke C. A., Strieter R. M. The role of cytokine networks in the local liver injury following hepatic ischemia/reperfusion in the rat. Hepatology. 1996 Mar;23(3):506–514. doi: 10.1002/hep.510230315. [DOI] [PubMed] [Google Scholar]
- Davenpeck K. L., Zagorski J., Schleimer R. P., Bochner B. S. Lipopolysaccharide-induced leukocyte rolling and adhesion in the rat mesenteric microcirculation: regulation by glucocorticoids and role of cytokines. J Immunol. 1998 Dec 15;161(12):6861–6870. [PubMed] [Google Scholar]
- Fan J., Marshall J. C., Jimenez M., Shek P. N., Zagorski J., Rotstein O. D. Hemorrhagic shock primes for increased expression of cytokine-induced neutrophil chemoattractant in the lung: role in pulmonary inflammation following lipopolysaccharide. J Immunol. 1998 Jul 1;161(1):440–447. [PubMed] [Google Scholar]
- Fink G. W., Norman J. G. Specific changes in the pancreatic expression of the interleukin 1 family of genes during experimental acute pancreatitis. Cytokine. 1997 Dec;9(12):1023–1027. doi: 10.1006/cyto.1997.0260. [DOI] [PubMed] [Google Scholar]
- Frossard J. L., Saluja A., Bhagat L., Lee H. S., Bhatia M., Hofbauer B., Steer M. L. The role of intercellular adhesion molecule 1 and neutrophils in acute pancreatitis and pancreatitis-associated lung injury. Gastroenterology. 1999 Mar;116(3):694–701. doi: 10.1016/s0016-5085(99)70192-7. [DOI] [PubMed] [Google Scholar]
- Fujimoto K., Hosotani R., Doi R., Wada M., Lee J. U., Koshiba T., Miyamoto Y., Imamura M. Role of neutrophils in cerulein-induced pancreatitis in rats: possible involvement of apoptosis. Digestion. 1997;58(5):421–430. doi: 10.1159/000201478. [DOI] [PubMed] [Google Scholar]
- Gerard C., Frossard J. L., Bhatia M., Saluja A., Gerard N. P., Lu B., Steer M. Targeted disruption of the beta-chemokine receptor CCR1 protects against pancreatitis-associated lung injury. J Clin Invest. 1997 Oct 15;100(8):2022–2027. doi: 10.1172/JCI119734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross V., Andreesen R., Leser H. G., Ceska M., Liehl E., Lausen M., Farthmann E. H., Schölmerich J. Interleukin-8 and neutrophil activation in acute pancreatitis. Eur J Clin Invest. 1992 Mar;22(3):200–203. doi: 10.1111/j.1365-2362.1992.tb01826.x. [DOI] [PubMed] [Google Scholar]
- Gukovskaya A. S., Gukovsky I., Zaninovic V., Song M., Sandoval D., Gukovsky S., Pandol S. J. Pancreatic acinar cells produce, release, and respond to tumor necrosis factor-alpha. Role in regulating cell death and pancreatitis. J Clin Invest. 1997 Oct 1;100(7):1853–1862. doi: 10.1172/JCI119714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haskill S., Peace A., Morris J., Sporn S. A., Anisowicz A., Lee S. W., Smith T., Martin G., Ralph P., Sager R. Identification of three related human GRO genes encoding cytokine functions. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7732–7736. doi: 10.1073/pnas.87.19.7732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirasawa N., Watanabe M., Mue S., Watanabe K., Tsurufuji S., Ohuchi K. Induction of neutrophil infiltration by rat chemotactic cytokine (CINC) and its inhibition by dexamethasone in rats. Inflammation. 1992 Apr;16(2):187–196. doi: 10.1007/BF00918958. [DOI] [PubMed] [Google Scholar]
- Hofbauer B., Saluja A. K., Bhatia M., Frossard J. L., Lee H. S., Bhagat L., Steer M. L. Effect of recombinant platelet-activating factor acetylhydrolase on two models of experimental acute pancreatitis. Gastroenterology. 1998 Nov;115(5):1238–1247. doi: 10.1016/s0016-5085(98)70096-4. [DOI] [PubMed] [Google Scholar]
- Inoue S., Nakao A., Kishimoto W., Murakami H., Itoh K., Itoh T., Harada A., Nonami T., Takagi H. Anti-neutrophil antibody attenuates the severity of acute lung injury in rats with experimental acute pancreatitis. Arch Surg. 1995 Jan;130(1):93–98. doi: 10.1001/archsurg.1995.01430010095020. [DOI] [PubMed] [Google Scholar]
- McKay C. J., Evans S., Sinclair M., Carter C. R., Imrie C. W. High early mortality rate from acute pancreatitis in Scotland, 1984-1995. Br J Surg. 1999 Oct;86(10):1302–1305. doi: 10.1046/j.1365-2168.1999.01246.x. [DOI] [PubMed] [Google Scholar]
- Nakagawa H., Komorita N., Shibata F., Ikesue A., Konishi K., Fujioka M., Kato H. Identification of cytokine-induced neutrophil chemoattractants (CINC), rat GRO/CINC-2 alpha and CINC-2 beta, produced by granulation tissue in culture: purification, complete amino acid sequences and characterization. Biochem J. 1994 Jul 15;301(Pt 2):545–550. doi: 10.1042/bj3010545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neoptolemos J. P., Raraty M., Finch M., Sutton R. Acute pancreatitis: the substantial human and financial costs. Gut. 1998 Jun;42(6):886–891. doi: 10.1136/gut.42.6.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman J. G., Fink G. W., Franz M. G. Acute pancreatitis induces intrapancreatic tumor necrosis factor gene expression. Arch Surg. 1995 Sep;130(9):966–970. doi: 10.1001/archsurg.1995.01430090052018. [DOI] [PubMed] [Google Scholar]
- Norman J., Franz M., Messina J., Riker A., Fabri P. J., Rosemurgy A. S., Gower W. R., Jr Interleukin-1 receptor antagonist decreases severity of experimental acute pancreatitis. Surgery. 1995 Jun;117(6):648–655. doi: 10.1016/s0039-6060(95)80008-5. [DOI] [PubMed] [Google Scholar]
- Osman M. O., Kristensen J. U., Jacobsen N. O., Lausten S. B., Deleuran B., Deleuran M., Gesser B., Matsushima K., Larsen C. G., Jensen S. L. A monoclonal anti-interleukin 8 antibody (WS-4) inhibits cytokine response and acute lung injury in experimental severe acute necrotising pancreatitis in rabbits. Gut. 1998 Aug;43(2):232–239. doi: 10.1136/gut.43.2.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoval D., Gukovskaya A., Reavey P., Gukovsky S., Sisk A., Braquet P., Pandol S. J., Poucell-Hatton S. The role of neutrophils and platelet-activating factor in mediating experimental pancreatitis. Gastroenterology. 1996 Oct;111(4):1081–1091. doi: 10.1016/s0016-5085(96)70077-x. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Suematsu M., Miura S., Liu Y. Y., Watanabe K., Miyasaka M., Tsurufuji S., Tsuchiya M. Rat CINC/gro: a novel mediator for locomotive and secretagogue activation of neutrophils in vivo. J Leukoc Biol. 1994 May;55(5):652–657. doi: 10.1002/jlb.55.5.652. [DOI] [PubMed] [Google Scholar]
- Watanabe K., Konishi K., Fujioka M., Kinoshita S., Nakagawa H. The neutrophil chemoattractant produced by the rat kidney epithelioid cell line NRK-52E is a protein related to the KC/gro protein. J Biol Chem. 1989 Nov 25;264(33):19559–19563. [PubMed] [Google Scholar]
- Werner J., Z'graggen K., Fernández-del Castillo C., Lewandrowski K. B., Compton C. C., Warshaw A. L. Specific therapy for local and systemic complications of acute pancreatitis with monoclonal antibodies against ICAM-1. Ann Surg. 1999 Jun;229(6):834–842. doi: 10.1097/00000658-199906000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittwer A. J., Carr L. S., Zagorski J., Dolecki G. J., Crippes B. A., De Larco J. E. High-level expression of cytokine-induced neutrophil chemoattractant (CINC) by a metastatic rat cell line: purification and production of blocking antibodies. J Cell Physiol. 1993 Aug;156(2):421–427. doi: 10.1002/jcp.1041560226. [DOI] [PubMed] [Google Scholar]
- Yamasaki Y., Matsuo Y., Zagorski J., Matsuura N., Onodera H., Itoyama Y., Kogure K. New therapeutic possibility of blocking cytokine-induced neutrophil chemoattractant on transient ischemic brain damage in rats. Brain Res. 1997 Jun 6;759(1):103–111. doi: 10.1016/s0006-8993(97)00251-5. [DOI] [PubMed] [Google Scholar]
- Yamasawa H., Ishii Y., Kitamura S. Cytokine-induced neutrophil chemoattractant in a rat model of lipopolysaccharide-induced acute lung injury. Inflammation. 1999 Jun;23(3):263–274. doi: 10.1023/a:1020278104132. [DOI] [PubMed] [Google Scholar]
- Zagorski J., Wahl S. M. Inhibition of acute peritoneal inflammation in rats by a cytokine-induced neutrophil chemoattractant receptor antagonist. J Immunol. 1997 Aug 1;159(3):1059–1062. [PubMed] [Google Scholar]


