Abstract
METHODS—Cellular localisation of the cyclooxygenase (COX) isozymes COX-1 and COX-2 was analysed in 24 cholangiocarcinomas, including 17 matched tissues originating from non-tumorous liver tissue adjacent to tumours and seven biopsies of normal human liver, by immunohistochemistry using isozyme selective antibodies. RESULTS—In normal liver, constitutive expression of COX-2 protein was a characteristic feature of hepatocytes whereas no COX-2 immunosignal was detectable in normal bile duct epithelium, Kupffer, and endothelial cells. In cholangiocarcinoma cells, COX-2 protein was strongly expressed at high frequency. The intensity, percentage of positive cells, and pattern of COX-2 expression were found to be independent of the stage of tumour differentiation. In hepatocytes of matched non-tumorous tissue, COX-2 expression was unaltered. In contrast, strong COX-1 expression was frequently localised to Kupffer cells, endothelial cells, and occasionally to hepatocytes, but not to bile duct epithelial cells. In approximately half of moderately and poorly differentiated but not well differentiated cholangiocarcinomas, weak to moderate COX-1 staining was found in tumour cells while COX-1 expression in Kupffer cells was much more pronounced. CONCLUSION—Aberrant COX-2 expression occurs during the early stage while COX-1 over expression seems to be related to later stages of cholangiocarcinogenesis. Keywords: cyclooxygenase; immunohistochemistry; cholangiocarcinoma; bile duct; hepatocyte; Kupffer cell
Full Text
The Full Text of this article is available as a PDF (238.9 KB).
Figure 1 .
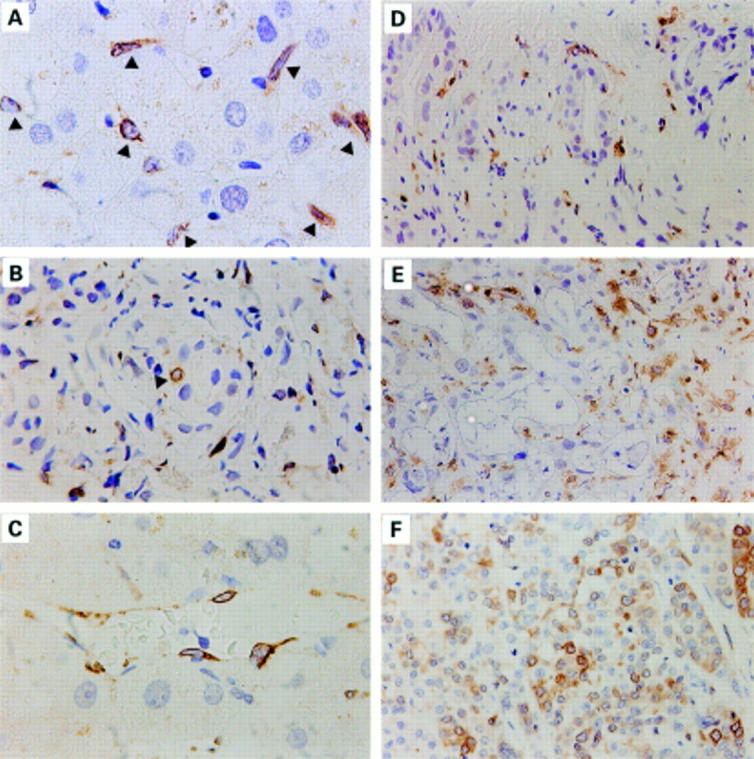
Immunohistochemical localisation of COX-1 in non-tumorous liver tissue and in intrahepatic cholangiocarcinomas at different stages of differentiation. Paraffin sections were stained using polyclonal goat anti-COX-1 antibodies (SC1754). The brown staining represents COX-1 protein. (A-C) Non-tumorous liver tissue. (A) Arrows indicate COX-1 positive Kupffer cells. (B) Arrow indicates a single epithelial cell of the bile duct expressing COX-1. (C) Blood vessel; endothelial cells express COX-1. (D) Well differentiated cholangiocarcinoma. (E) Moderately and (F) poorly differentiated cholangiocarcinomas showing COX-1 immunosignals in epithelial cells of the gland-like structures. Original magnifications: 1:375 (B, D, E, F); 1:725 (A, C).
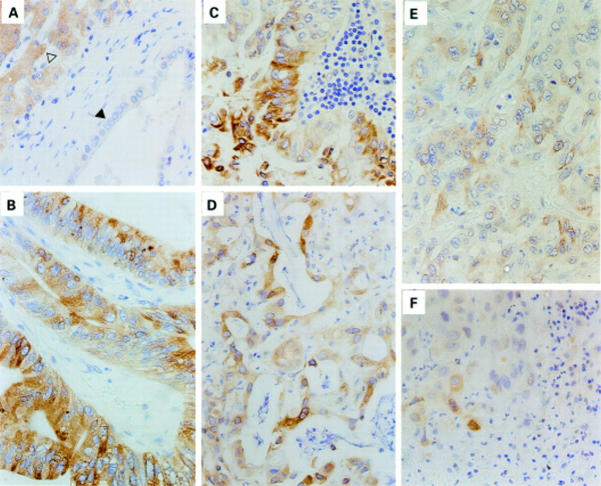
Figure 2 .
Immunohistochemical localisation of COX-2 in non-tumorous liver tissue and in intrahepatic cholangiocarcinomas at different stages of differentiation. Paraffin sections were stained using the polyclonal goat anti-COX-2 antibodies (SC1745). (A) Non-tumorous tissue demonstrating positive COX-2 immunosignals in hepatocytes (open arrow) but not in bile duct epithelial cells (filled arrow). (B, C) Well differentiated, (D) moderately differentiated, and (E, F) poorly differentiated cholangiocarcinomas showing COX-2 immunosignals in epithelial cells of the gland-like structures. Original magnifications: 1:375 (A-F).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes C. J., Lee M. Chemoprevention of spontaneous intestinal adenomas in the adenomatous polyposis coli Min mouse model with aspirin. Gastroenterology. 1998 May;114(5):873–877. doi: 10.1016/s0016-5085(98)70305-1. [DOI] [PubMed] [Google Scholar]
- Boolbol S. K., Dannenberg A. J., Chadburn A., Martucci C., Guo X. J., Ramonetti J. T., Abreu-Goris M., Newmark H. L., Lipkin M. L., DeCosse J. J. Cyclooxygenase-2 overexpression and tumor formation are blocked by sulindac in a murine model of familial adenomatous polyposis. Cancer Res. 1996 Jun 1;56(11):2556–2560. [PubMed] [Google Scholar]
- Buckman S. Y., Gresham A., Hale P., Hruza G., Anast J., Masferrer J., Pentland A. P. COX-2 expression is induced by UVB exposure in human skin: implications for the development of skin cancer. Carcinogenesis. 1998 May;19(5):723–729. doi: 10.1093/carcin/19.5.723. [DOI] [PubMed] [Google Scholar]
- Chan G., Boyle J. O., Yang E. K., Zhang F., Sacks P. G., Shah J. P., Edelstein D., Soslow R. A., Koki A. T., Woerner B. M. Cyclooxygenase-2 expression is up-regulated in squamous cell carcinoma of the head and neck. Cancer Res. 1999 Mar 1;59(5):991–994. [PubMed] [Google Scholar]
- Dubois R. N., Abramson S. B., Crofford L., Gupta R. A., Simon L. S., Van De Putte L. B., Lipsky P. E. Cyclooxygenase in biology and disease. FASEB J. 1998 Sep;12(12):1063–1073. [PubMed] [Google Scholar]
- Eberhart C. E., Coffey R. J., Radhika A., Giardiello F. M., Ferrenbach S., DuBois R. N. Up-regulation of cyclooxygenase 2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology. 1994 Oct;107(4):1183–1188. doi: 10.1016/0016-5085(94)90246-1. [DOI] [PubMed] [Google Scholar]
- Fürstenberger G., Gross M., Marks F. Eicosanoids and multistage carcinogenesis in NMRI mouse skin: role of prostaglandins E and F in conversion (first stage of tumor promotion) and promotion (second stage of tumor promotion). Carcinogenesis. 1989 Jan;10(1):91–96. doi: 10.1093/carcin/10.1.91. [DOI] [PubMed] [Google Scholar]
- Giardiello F. M., Hamilton S. R., Krush A. J., Piantadosi S., Hylind L. M., Celano P., Booker S. V., Robinson C. R., Offerhaus G. J. Treatment of colonic and rectal adenomas with sulindac in familial adenomatous polyposis. N Engl J Med. 1993 May 6;328(18):1313–1316. doi: 10.1056/NEJM199305063281805. [DOI] [PubMed] [Google Scholar]
- Hao X., Bishop A. E., Wallace M., Wang H., Willcocks T. C., Maclouf J., Polak J. M., Knight S., Talbot I. C. Early expression of cyclo-oxygenase-2 during sporadic colorectal carcinogenesis. J Pathol. 1999 Feb;187(3):295–301. doi: 10.1002/(SICI)1096-9896(199902)187:3<295::AID-PATH254>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Hwang D., Scollard D., Byrne J., Levine E. Expression of cyclooxygenase-1 and cyclooxygenase-2 in human breast cancer. J Natl Cancer Inst. 1998 Mar 18;90(6):455–460. doi: 10.1093/jnci/90.6.455. [DOI] [PubMed] [Google Scholar]
- Jacoby R. F., Marshall D. J., Newton M. A., Novakovic K., Tutsch K., Cole C. E., Lubet R. A., Kelloff G. J., Verma A., Moser A. R. Chemoprevention of spontaneous intestinal adenomas in the Apc Min mouse model by the nonsteroidal anti-inflammatory drug piroxicam. Cancer Res. 1996 Feb 15;56(4):710–714. [PubMed] [Google Scholar]
- Kalgutkar A. S., Crews B. C., Rowlinson S. W., Garner C., Seibert K., Marnett L. J. Aspirin-like molecules that covalently inactivate cyclooxygenase-2. Science. 1998 May 22;280(5367):1268–1270. doi: 10.1126/science.280.5367.1268. [DOI] [PubMed] [Google Scholar]
- Kargman S. L., O'Neill G. P., Vickers P. J., Evans J. F., Mancini J. A., Jothy S. Expression of prostaglandin G/H synthase-1 and -2 protein in human colon cancer. Cancer Res. 1995 Jun 15;55(12):2556–2559. [PubMed] [Google Scholar]
- Kawamori T., Rao C. V., Seibert K., Reddy B. S. Chemopreventive activity of celecoxib, a specific cyclooxygenase-2 inhibitor, against colon carcinogenesis. Cancer Res. 1998 Feb 1;58(3):409–412. [PubMed] [Google Scholar]
- Koga H., Sakisaka S., Ohishi M., Kawaguchi T., Taniguchi E., Sasatomi K., Harada M., Kusaba T., Tanaka M., Kimura R. Expression of cyclooxygenase-2 in human hepatocellular carcinoma: relevance to tumor dedifferentiation. Hepatology. 1999 Mar;29(3):688–696. doi: 10.1002/hep.510290355. [DOI] [PubMed] [Google Scholar]
- Lowenfels A. B. Opisthorchis viverrini infection and cholangiocarcinoma. Gastroenterology. 1985 Dec;89(6):1449–1449. doi: 10.1016/0016-5085(85)90686-9. [DOI] [PubMed] [Google Scholar]
- Marks F., Fürstenberger G. Proliferative responses of the skin to external stimuli. Environ Health Perspect. 1993 Dec;101 (Suppl 5):95–101. doi: 10.1289/ehp.93101s595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Decker K., Kopp-Schneider A., Marks F., Seibert K., Fürstenberger G. Localization of prostaglandin H synthase isoenzymes in murine epidermal tumors: suppression of skin tumor promotion by inhibition of prostaglandin H synthase-2. Mol Carcinog. 1998 Sep;23(1):36–44. doi: 10.1002/(sici)1098-2744(199809)23:1<36::aid-mc5>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Müller-Decker K., Reinerth G., Krieg P., Zimmermann R., Heise H., Bayerl C., Marks F., Fürstenberger G. Prostaglandin-H-synthase isozyme expression in normal and neoplastic human skin. Int J Cancer. 1999 Aug 27;82(5):648–656. doi: 10.1002/(sici)1097-0215(19990827)82:5<648::aid-ijc6>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Müller-Decker K., Scholz K., Neufang G., Marks F., Fürstenberger G. Localization of prostaglandin-H synthase-1 and -2 in mouse skin: implications for cutaneous function. Exp Cell Res. 1998 Jul 10;242(1):84–91. doi: 10.1006/excr.1998.4068. [DOI] [PubMed] [Google Scholar]
- Narko K., Ristimäki A., MacPhee M., Smith E., Haudenschild C. C., Hla T. Tumorigenic transformation of immortalized ECV endothelial cells by cyclooxygenase-1 overexpression. J Biol Chem. 1997 Aug 22;272(34):21455–21460. doi: 10.1074/jbc.272.34.21455. [DOI] [PubMed] [Google Scholar]
- O'Neill G. P., Ford-Hutchinson A. W. Expression of mRNA for cyclooxygenase-1 and cyclooxygenase-2 in human tissues. FEBS Lett. 1993 Sep 13;330(2):156–160. doi: 10.1016/0014-5793(93)80263-t. [DOI] [PubMed] [Google Scholar]
- Oshima M., Dinchuk J. E., Kargman S. L., Oshima H., Hancock B., Kwong E., Trzaskos J. M., Evans J. F., Taketo M. M. Suppression of intestinal polyposis in Apc delta716 knockout mice by inhibition of cyclooxygenase 2 (COX-2). Cell. 1996 Nov 29;87(5):803–809. doi: 10.1016/s0092-8674(00)81988-1. [DOI] [PubMed] [Google Scholar]
- Pairojkul C., Shirai T., Hirohashi S., Thamavit W., Bhudhisawat W., Uttaravicien T., Itoh M., Ito N. Multistage carcinogenesis of liver-fluke-associated cholangiocarcinoma in Thailand. Princess Takamatsu Symp. 1991;22:77–86. [PubMed] [Google Scholar]
- Parkin D. M., Srivatanakul P., Khlat M., Chenvidhya D., Chotiwan P., Insiripong S., L'Abbé K. A., Wild C. P. Liver cancer in Thailand. I. A case-control study of cholangiocarcinoma. Int J Cancer. 1991 May 30;48(3):323–328. doi: 10.1002/ijc.2910480302. [DOI] [PubMed] [Google Scholar]
- Rao C. V., Rivenson A., Simi B., Zang E., Kelloff G., Steele V., Reddy B. S. Chemoprevention of colon carcinogenesis by sulindac, a nonsteroidal anti-inflammatory agent. Cancer Res. 1995 Apr 1;55(7):1464–1472. [PubMed] [Google Scholar]
- Ristimäki A., Honkanen N., Jänkälä H., Sipponen P., Härkönen M. Expression of cyclooxygenase-2 in human gastric carcinoma. Cancer Res. 1997 Apr 1;57(7):1276–1280. [PubMed] [Google Scholar]
- Sano H., Kawahito Y., Wilder R. L., Hashiramoto A., Mukai S., Asai K., Kimura S., Kato H., Kondo M., Hla T. Expression of cyclooxygenase-1 and -2 in human colorectal cancer. Cancer Res. 1995 Sep 1;55(17):3785–3789. [PubMed] [Google Scholar]
- Shiota G., Okubo M., Noumi T., Noguchi N., Oyama K., Takano Y., Yashima K., Kishimoto Y., Kawasaki H. Cyclooxygenase-2 expression in hepatocellular carcinoma. Hepatogastroenterology. 1999 Jan-Feb;46(25):407–412. [PubMed] [Google Scholar]
- Sinawat P., Hemsrichart V. A histopathologic study of 61 cases of peripheral intrahepatic cholangiocarcinoma. J Med Assoc Thai. 1991 Oct;74(10):448–453. [PubMed] [Google Scholar]
- Smalley W. E., DuBois R. N. Colorectal cancer and nonsteroidal anti-inflammatory drugs. Adv Pharmacol. 1997;39:1–20. doi: 10.1016/s1054-3589(08)60067-8. [DOI] [PubMed] [Google Scholar]
- Smith C. J., Zhang Y., Koboldt C. M., Muhammad J., Zweifel B. S., Shaffer A., Talley J. J., Masferrer J. L., Seibert K., Isakson P. C. Pharmacological analysis of cyclooxygenase-1 in inflammation. Proc Natl Acad Sci U S A. 1998 Oct 27;95(22):13313–13318. doi: 10.1073/pnas.95.22.13313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thamavit W., Pairojkul C., Tiwawech D., Shirai T., Ito N. Strong promoting effect of Opisthorchis viverrini infection on dimethylnitrosamine-initiated hamster liver. Cancer Lett. 1994 Apr 1;78(1-3):121–125. doi: 10.1016/0304-3835(94)90040-x. [DOI] [PubMed] [Google Scholar]
- Tucker O. N., Dannenberg A. J., Yang E. K., Zhang F., Teng L., Daly J. M., Soslow R. A., Masferrer J. L., Woerner B. M., Koki A. T. Cyclooxygenase-2 expression is up-regulated in human pancreatic cancer. Cancer Res. 1999 Mar 1;59(5):987–990. [PubMed] [Google Scholar]
- Vane J. R., Bakhle Y. S., Botting R. M. Cyclooxygenases 1 and 2. Annu Rev Pharmacol Toxicol. 1998;38:97–120. doi: 10.1146/annurev.pharmtox.38.1.97. [DOI] [PubMed] [Google Scholar]
- Winde G., Schmid K. W., Schlegel W., Fischer R., Osswald H., Bünte H. Complete reversion and prevention of rectal adenomas in colectomized patients with familial adenomatous polyposis by rectal low-dose sulindac maintenance treatment. Advantages of a low-dose nonsteroidal anti-inflammatory drug regimen in reversing adenomas exceeding 33 months. Dis Colon Rectum. 1995 Aug;38(8):813–830. doi: 10.1007/BF02049838. [DOI] [PubMed] [Google Scholar]
- Wolff H., Saukkonen K., Anttila S., Karjalainen A., Vainio H., Ristimäki A. Expression of cyclooxygenase-2 in human lung carcinoma. Cancer Res. 1998 Nov 15;58(22):4997–5001. [PubMed] [Google Scholar]
- Zimmermann K. C., Sarbia M., Weber A. A., Borchard F., Gabbert H. E., Schrör K. Cyclooxygenase-2 expression in human esophageal carcinoma. Cancer Res. 1999 Jan 1;59(1):198–204. [PubMed] [Google Scholar]