Abstract
BACKGROUND/AIMS—Vascular endothelial growth factor (VEGF) plays a key role in regulation of tumour associated angiogenesis. In the current study we analysed expression of VEGF and its receptors in human hepatocellular carcinoma (HCC) and investigated the molecular mechanisms of VEGF regulation by hypoxia. METHODS—VEGF, kinase domain region (KDR)/fetal liver kinase 1 (flk-1), and flt-1 expression were examined by immunohistochemistry and in situ hybridisation in 15 human HCC tissues. Expression of VEGF and regulation by hypoxia were assessed in three human HCC cell lines using a quantitative competitive reverse transcription-polymerase chain reaction, ELISA, and a series of 5' deletion reporter gene constructs of the human VEGF promoter in transient transfection assays. RESULTS—We observed over expression of VEGF mRNA and protein in HCC compared with cirrhosis or normal liver. Expression of VEGF in tumour cells was strongly increased in areas directly adjacent to necrotic/hypoxic regions. Both VEGF receptors were detected in vascular endothelia of HCC while only KDR/flk-1 receptors were detected in endothelial cells of cirrhotic livers. Expression of VEGF was observed in all human HCC cell lines examined. Hypoxia (1% oxygen) resulted in profound upregulation of VEGF mRNA and protein levels. Furthermore, hypoxia treatment resulted in a doubling of VEGF mRNA stability. Deletion analysis of the human VEGF 5' flanking region −2018 and +50 demonstrated induction of VEGF promoter activity under hypoxic conditions which was significantly decreased following deletion of the region −1286 and −789 suggesting a substantial contribution of the −975 putative hypoxia inducible factor 1 binding site to hypoxia mediated transcriptional activation of the VEGF gene. CONCLUSION—These data suggest hypoxia as a central stimulus of angiogenesis in human HCC through upregulation of VEGF gene expression by at least two distinct molecular mechanisms: activation of VEGF gene transcription and an increase in VEGF mRNA stability. Keywords: hepatocellular carcinoma; angiogenesis; vascular endothelial growth factor; hypoxia
Full Text
The Full Text of this article is available as a PDF (331.3 KB).
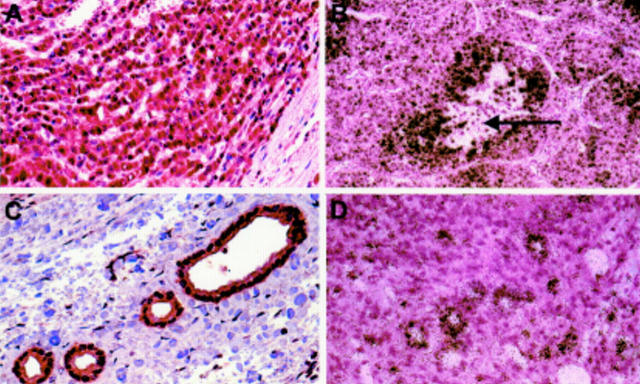
Figure 1 .
Expression of vascular endothelial growth factor (VEGF) in human hepatocellular carcinoma (HCC). Immunostaining with polyclonal VEGF antibody (A, C) and in situ hybridisation with 35S labelled antisense cRNA for VEGF (B, D). VEGF was expressed by HCC cells (A, B) and epithelial cells of bile ducts (C, D). Exposure time for autoradiography was 14 days. The arrow indicates necrosis. Original magnification: A, C, and D ×40; B ×20.
Figure 2 .
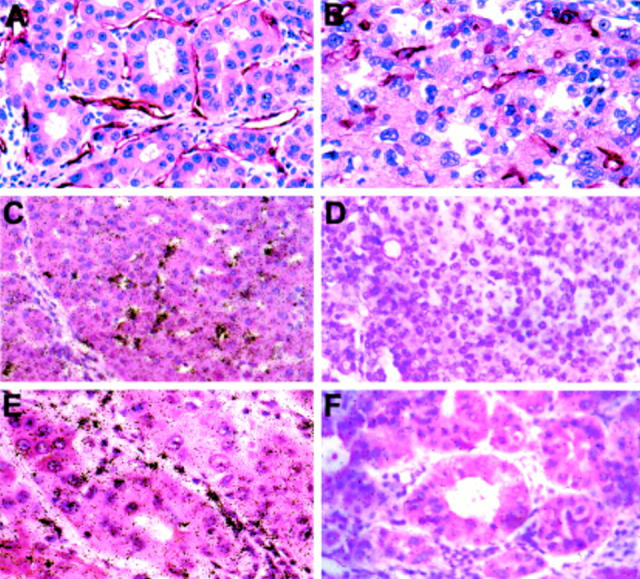
Expression of vascular endothelial growth factor (VEGF) receptors kinase domain region (KDR)/fetal liver kinase 1 (flk-1) and fms-like tyrosine kinase 1 (flt-1) in human hepatocellular carcinoma (HCC). Immunostaining with anti-CD31 antibody (A) and anti-flt-1 (B). In situ hybridisation with 35S labelled antisense cRNA for flt-1 (C) and KDR/flk-1 (E) or sense probes, respectively (D, F). Flt-1 (B, C) and KDR/flk-1 (E) expression was observed exclusively in endothelial cells of HCC tissue. Hybridisation with sense cRNAs for flt-1 (D) and KDR/flk-1 (F) did not reveal any significant hybridisation signal. Exposure time for autoradiography was 14 days. Original magnification: A, B, E, and F ×40; C and D ×20.
Figure 3 .
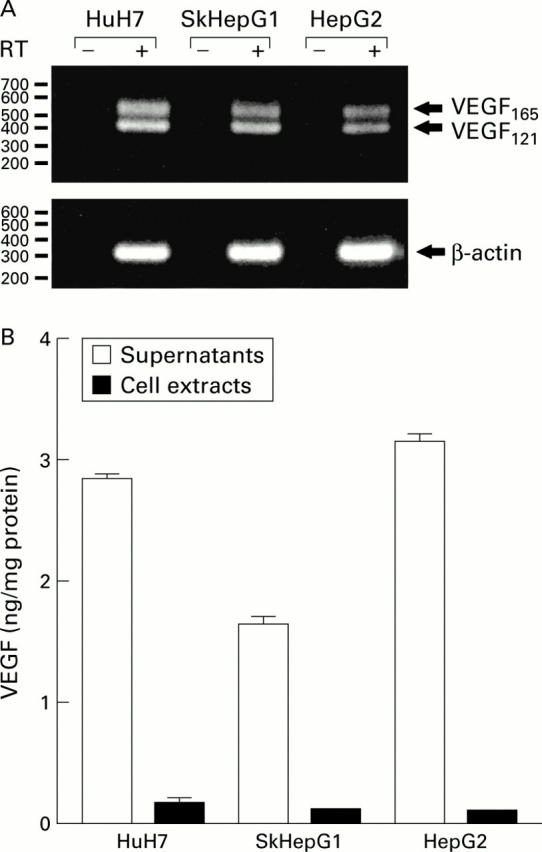
Expression of vascular endothelial growth factor (VEGF) in human hepatocellular carcinoma (HCC) cell lines. (A) Analysis of VEGF mRNA transcripts by reverse transcription-polymerase chain reaction (RT-PCR) using primers specific for all known isoforms. The size of the indicated bands of 403 and 535 bp was determined by a 100 bp ladder and correspond to VEGF121 and VEGF165, respectively. RT−, PCR without reverse transcription. (B) Expression of VEGF protein: a VEGF specific ELISA was used to determine VEGF concentrations in supernatants and cell extracts of the indicated cell lines and normalised to protein content. Mean (SEM) of three experiments, each performed in triplicate.
Figure 4 .
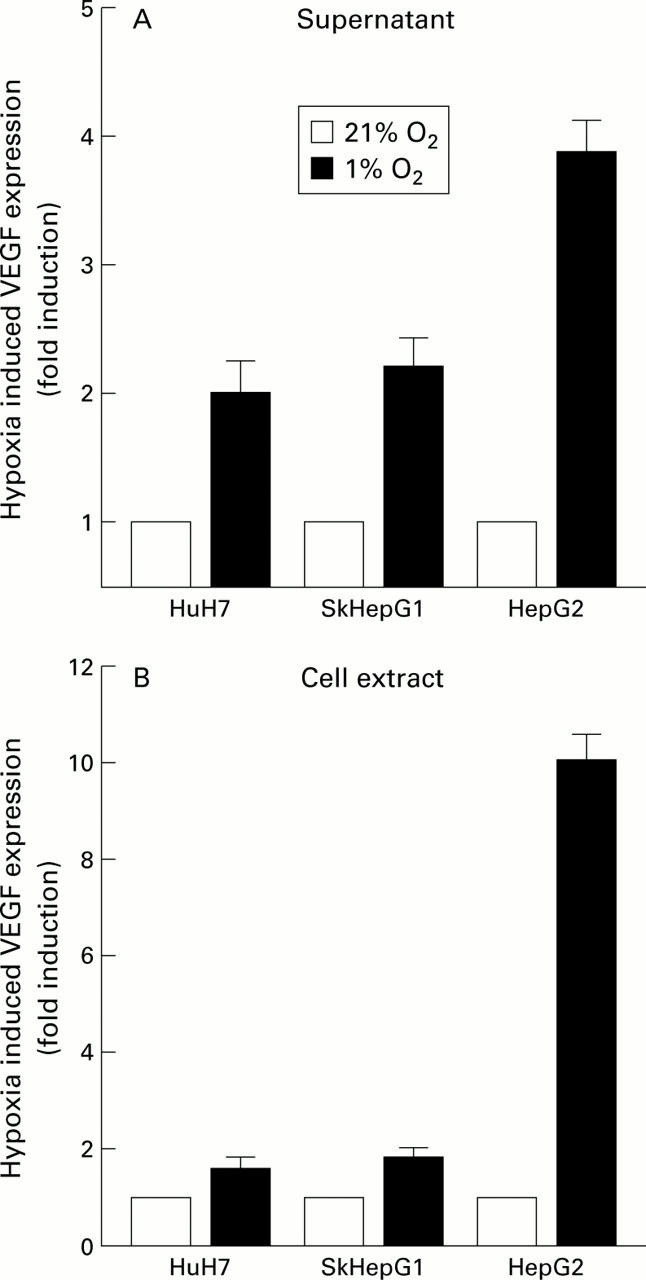
Hypoxia mediated induction of vascular endothelial growth factor (VEGF) protein in human hepatocellular carcinoma (HCC) cell lines. VEGF specific ELISA was used to determine VEGF concentrations in supernatants (A) and cell extracts (B) of the indicated cell lines incubated either under normoxic (21% O2 ) or hypoxic (1% O2 ) conditions for 16 hours and normalised to protein content. Mean (SEM) of three experiments, each performed in triplicate.
Figure 5 .
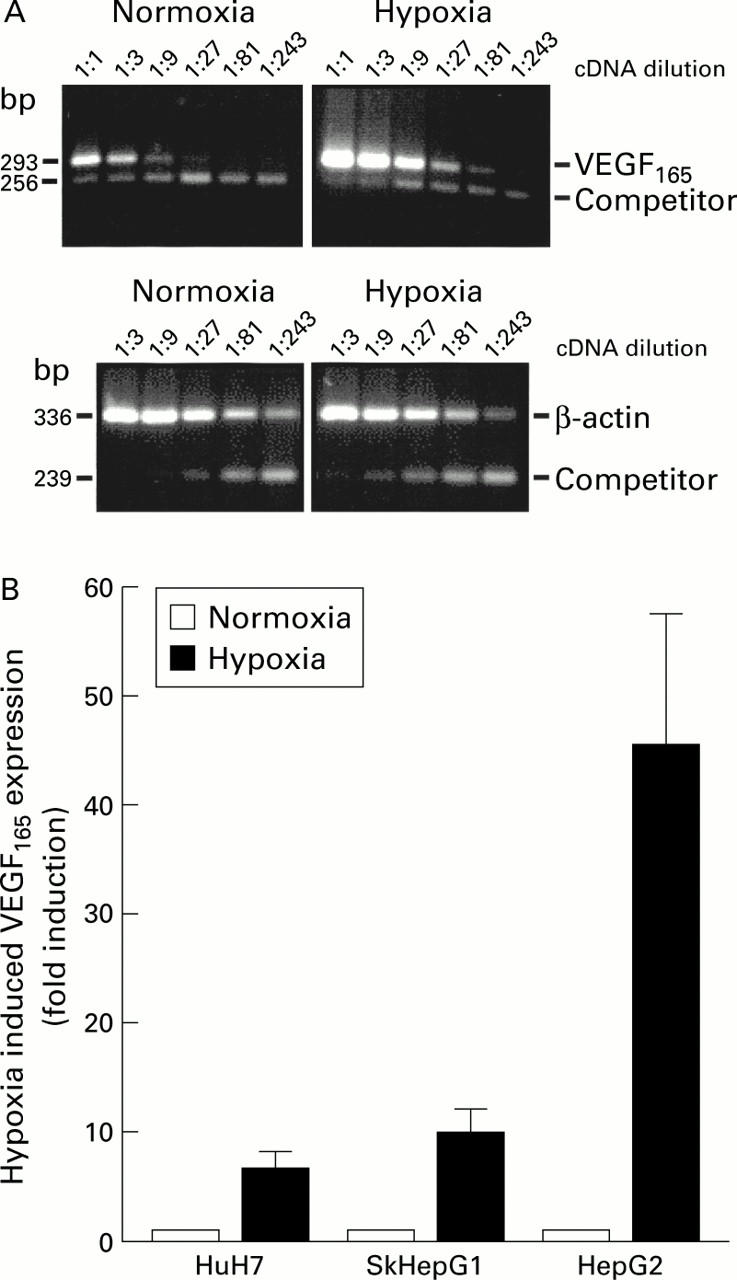
Effects of hypoxia on vascular endothelial growth factor (VEGF) mRNA expression in hepatocellular carcinoma (HCC) cell lines. VEGF mRNA expression was measured by quantitative competitive reverse transcription-polymerase chain reaction (RT-PCR). Total RNA was isolated from cells incubated for 16 hours under normoxic or hypoxic conditions and subjected to RT. A constant amount of competitor and threefold serial dilutions of cDNAs were used as templates for PCR using VEGF165 or β-actin specific primers. (A) Representative image of ethidium bromide stained gels for HepG2 cells. (B) Quantitative analysis. VEGF165 expression was calculated as the molar ratio of VEGF to β-actin copies. Mean (SEM) values of three independent experiments determined as fold induction by hypoxia.
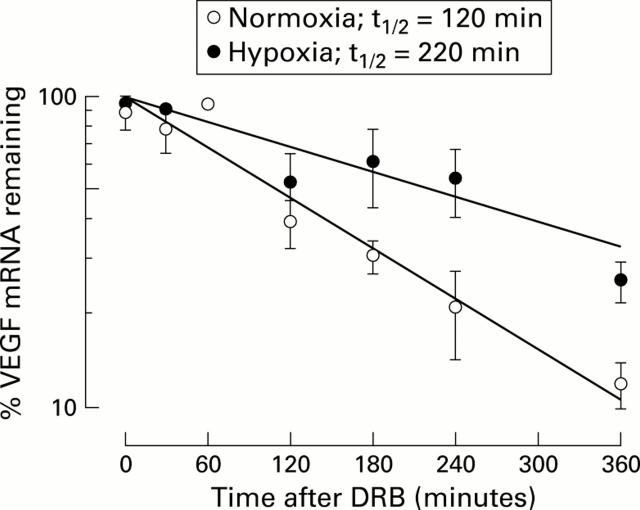
Figure 6 .
Hypoxia increased vascular endothelial growth factor (VEGF) mRNA half life in HepG2 cells. Cells were incubated for 16 hours under normoxic or hypoxic conditions and subsequently incubated with 150 µM DRB to block transcription. Cells were harvested for RNA after 0, 30, 60, 120, 180, 240, and 360 minutes. VEGF mRNA was then determined by quantitative competitive reverse transcription-polymerase chain reaction as described in materials and methods. Mean (SEM) values of three independent experiments.
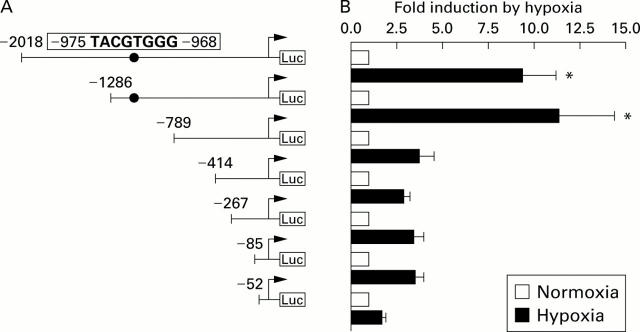
Figure 7 .
Effects of hypoxia on vascular endothelial growth factor (VEGF) gene transcription. (A) Schematic diagram of the VEGF promoter-Luc reporter constructs. The nucleotide sequence -2018 to +50 bp of the human VEGF gene promoter was cloned in front of a luciferase gene, and serial 5' deletion constructs were generated between positions −2018 and −52 bp. The transcription start is indicated by an arrow. Potential consensus binding sites for HIF-1 are indicated (•). (B) Hypoxia mediated transactivation in HepG2 cells. HepG2 cells were transiently cotransfected with VEGF-Luc and β-gal plasmids and incubated for 16 hours with 21% O2 or 1% O2. Luciferase activity was determined and normalised for transfection efficiency. Mean (SEM) values of four independent experiments, each performed in triplicate, and determined as fold induction by hypoxia.*Significantly different from control (p<0.001).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barleon B., Hauser S., Schöllmann C., Weindel K., Marmé D., Yayon A., Weich H. A. Differential expression of the two VEGF receptors flt and KDR in placenta and vascular endothelial cells. J Cell Biochem. 1994 Jan;54(1):56–66. doi: 10.1002/jcb.240540107. [DOI] [PubMed] [Google Scholar]
- Breier G., Clauss M., Risau W. Coordinate expression of vascular endothelial growth factor receptor-1 (flt-1) and its ligand suggests a paracrine regulation of murine vascular development. Dev Dyn. 1995 Nov;204(3):228–239. doi: 10.1002/aja.1002040303. [DOI] [PubMed] [Google Scholar]
- Brown L. F., Berse B., Jackman R. W., Tognazzi K., Guidi A. J., Dvorak H. F., Senger D. R., Connolly J. L., Schnitt S. J. Expression of vascular permeability factor (vascular endothelial growth factor) and its receptors in breast cancer. Hum Pathol. 1995 Jan;26(1):86–91. doi: 10.1016/0046-8177(95)90119-1. [DOI] [PubMed] [Google Scholar]
- Brown L. F., Berse B., Jackman R. W., Tognazzi K., Manseau E. J., Dvorak H. F., Senger D. R. Increased expression of vascular permeability factor (vascular endothelial growth factor) and its receptors in kidney and bladder carcinomas. Am J Pathol. 1993 Nov;143(5):1255–1262. [PMC free article] [PubMed] [Google Scholar]
- Brown L. F., Berse B., Jackman R. W., Tognazzi K., Manseau E. J., Senger D. R., Dvorak H. F. Expression of vascular permeability factor (vascular endothelial growth factor) and its receptors in adenocarcinomas of the gastrointestinal tract. Cancer Res. 1993 Oct 1;53(19):4727–4735. [PubMed] [Google Scholar]
- Claffey K. P., Shih S. C., Mullen A., Dziennis S., Cusick J. L., Abrams K. R., Lee S. W., Detmar M. Identification of a human VPF/VEGF 3' untranslated region mediating hypoxia-induced mRNA stability. Mol Biol Cell. 1998 Feb;9(2):469–481. doi: 10.1091/mbc.9.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara N., Heinsohn H., Walder C. E., Bunting S., Thomas G. R. The regulation of blood vessel growth by vascular endothelial growth factor. Ann N Y Acad Sci. 1995 Mar 27;752:246–256. doi: 10.1111/j.1749-6632.1995.tb17435.x. [DOI] [PubMed] [Google Scholar]
- Ferrara N. The role of vascular endothelial growth factor in pathological angiogenesis. Breast Cancer Res Treat. 1995;36(2):127–137. doi: 10.1007/BF00666035. [DOI] [PubMed] [Google Scholar]
- Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995 Jan;1(1):27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- Folkman J. Seminars in Medicine of the Beth Israel Hospital, Boston. Clinical applications of research on angiogenesis. N Engl J Med. 1995 Dec 28;333(26):1757–1763. doi: 10.1056/NEJM199512283332608. [DOI] [PubMed] [Google Scholar]
- Folkman J., Watson K., Ingber D., Hanahan D. Induction of angiogenesis during the transition from hyperplasia to neoplasia. Nature. 1989 May 4;339(6219):58–61. doi: 10.1038/339058a0. [DOI] [PubMed] [Google Scholar]
- Forsythe J. A., Jiang B. H., Iyer N. V., Agani F., Leung S. W., Koos R. D., Semenza G. L. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996 Sep;16(9):4604–4613. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D., Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996 Aug 9;86(3):353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- Houck K. A., Ferrara N., Winer J., Cachianes G., Li B., Leung D. W. The vascular endothelial growth factor family: identification of a fourth molecular species and characterization of alternative splicing of RNA. Mol Endocrinol. 1991 Dec;5(12):1806–1814. doi: 10.1210/mend-5-12-1806. [DOI] [PubMed] [Google Scholar]
- Houck K. A., Leung D. W., Rowland A. M., Winer J., Ferrara N. Dual regulation of vascular endothelial growth factor bioavailability by genetic and proteolytic mechanisms. J Biol Chem. 1992 Dec 25;267(36):26031–26037. [PubMed] [Google Scholar]
- Huang L. E., Arany Z., Livingston D. M., Bunn H. F. Activation of hypoxia-inducible transcription factor depends primarily upon redox-sensitive stabilization of its alpha subunit. J Biol Chem. 1996 Dec 13;271(50):32253–32259. doi: 10.1074/jbc.271.50.32253. [DOI] [PubMed] [Google Scholar]
- Ikeda E., Achen M. G., Breier G., Risau W. Hypoxia-induced transcriptional activation and increased mRNA stability of vascular endothelial growth factor in C6 glioma cells. J Biol Chem. 1995 Aug 25;270(34):19761–19766. doi: 10.1074/jbc.270.34.19761. [DOI] [PubMed] [Google Scholar]
- Jin C. F., Mata M., Fink D. J. Rapid construction of deleted DNA fragments for use as internal standards in competitive PCR. PCR Methods Appl. 1994 Feb;3(4):252–255. doi: 10.1101/gr.3.4.252. [DOI] [PubMed] [Google Scholar]
- Kim K. J., Li B., Winer J., Armanini M., Gillett N., Phillips H. S., Ferrara N. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature. 1993 Apr 29;362(6423):841–844. doi: 10.1038/362841a0. [DOI] [PubMed] [Google Scholar]
- Kim K. W., Bae S. K., Lee O. H., Bae M. H., Lee M. J., Park B. C. Insulin-like growth factor II induced by hypoxia may contribute to angiogenesis of human hepatocellular carcinoma. Cancer Res. 1998 Jan 15;58(2):348–351. [PubMed] [Google Scholar]
- Kolch W., Martiny-Baron G., Kieser A., Marmé D. Regulation of the expression of the VEGF/VPS and its receptors: role in tumor angiogenesis. Breast Cancer Res Treat. 1995;36(2):139–155. doi: 10.1007/BF00666036. [DOI] [PubMed] [Google Scholar]
- Kondo S., Asano M., Suzuki H. Significance of vascular endothelial growth factor/vascular permeability factor for solid tumor growth, and its inhibition by the antibody. Biochem Biophys Res Commun. 1993 Aug 16;194(3):1234–1241. doi: 10.1006/bbrc.1993.1955. [DOI] [PubMed] [Google Scholar]
- Leung D. W., Cachianes G., Kuang W. J., Goeddel D. V., Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989 Dec 8;246(4935):1306–1309. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- Levy A. P., Levy N. S., Goldberg M. A. Post-transcriptional regulation of vascular endothelial growth factor by hypoxia. J Biol Chem. 1996 Feb 2;271(5):2746–2753. doi: 10.1074/jbc.271.5.2746. [DOI] [PubMed] [Google Scholar]
- Levy A. P., Levy N. S., Wegner S., Goldberg M. A. Transcriptional regulation of the rat vascular endothelial growth factor gene by hypoxia. J Biol Chem. 1995 Jun 2;270(22):13333–13340. doi: 10.1074/jbc.270.22.13333. [DOI] [PubMed] [Google Scholar]
- Levy N. S., Chung S., Furneaux H., Levy A. P. Hypoxic stabilization of vascular endothelial growth factor mRNA by the RNA-binding protein HuR. J Biol Chem. 1998 Mar 13;273(11):6417–6423. doi: 10.1074/jbc.273.11.6417. [DOI] [PubMed] [Google Scholar]
- Matthes H., Kaiser A., Stier U., Riecken E. O., Rosewicz S. Glucocorticoid receptor gene expression in the exocrine and endocrine rat pancreas. Endocrinology. 1994 Jul;135(1):476–479. doi: 10.1210/endo.135.1.8013388. [DOI] [PubMed] [Google Scholar]
- Matthews W., Jordan C. T., Gavin M., Jenkins N. A., Copeland N. G., Lemischka I. R. A receptor tyrosine kinase cDNA isolated from a population of enriched primitive hematopoietic cells and exhibiting close genetic linkage to c-kit. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):9026–9030. doi: 10.1073/pnas.88.20.9026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millauer B., Longhi M. P., Plate K. H., Shawver L. K., Risau W., Ullrich A., Strawn L. M. Dominant-negative inhibition of Flk-1 suppresses the growth of many tumor types in vivo. Cancer Res. 1996 Apr 1;56(7):1615–1620. [PubMed] [Google Scholar]
- Millauer B., Shawver L. K., Plate K. H., Risau W., Ullrich A. Glioblastoma growth inhibited in vivo by a dominant-negative Flk-1 mutant. Nature. 1994 Feb 10;367(6463):576–579. doi: 10.1038/367576a0. [DOI] [PubMed] [Google Scholar]
- Millauer B., Wizigmann-Voos S., Schnürch H., Martinez R., Møller N. P., Risau W., Ullrich A. High affinity VEGF binding and developmental expression suggest Flk-1 as a major regulator of vasculogenesis and angiogenesis. Cell. 1993 Mar 26;72(6):835–846. doi: 10.1016/0092-8674(93)90573-9. [DOI] [PubMed] [Google Scholar]
- Mise M., Arii S., Higashituji H., Furutani M., Niwano M., Harada T., Ishigami S., Toda Y., Nakayama H., Fukumoto M. Clinical significance of vascular endothelial growth factor and basic fibroblast growth factor gene expression in liver tumor. Hepatology. 1996 Mar;23(3):455–464. doi: 10.1053/jhep.1996.v23.pm0008617424. [DOI] [PubMed] [Google Scholar]
- Miura H., Miyazaki T., Kuroda M., Oka T., Machinami R., Kodama T., Shibuya M., Makuuchi M., Yazaki Y., Ohnishi S. Increased expression of vascular endothelial growth factor in human hepatocellular carcinoma. J Hepatol. 1997 Nov;27(5):854–861. doi: 10.1016/s0168-8278(97)80323-6. [DOI] [PubMed] [Google Scholar]
- Park B. C., Huh M. H., Seo J. H. Differential expression of transforming growth factor alpha and insulin-like growth factor II in chronic active hepatitis B, cirrhosis and hepatocellular carcinoma. J Hepatol. 1995 Mar;22(3):286–294. doi: 10.1016/0168-8278(95)80281-9. [DOI] [PubMed] [Google Scholar]
- Plate K. H., Breier G., Weich H. A., Mennel H. D., Risau W. Vascular endothelial growth factor and glioma angiogenesis: coordinate induction of VEGF receptors, distribution of VEGF protein and possible in vivo regulatory mechanisms. Int J Cancer. 1994 Nov 15;59(4):520–529. doi: 10.1002/ijc.2910590415. [DOI] [PubMed] [Google Scholar]
- Plate K. H., Breier G., Weich H. A., Risau W. Vascular endothelial growth factor is a potential tumour angiogenesis factor in human gliomas in vivo. Nature. 1992 Oct 29;359(6398):845–848. doi: 10.1038/359845a0. [DOI] [PubMed] [Google Scholar]
- Poltorak Z., Cohen T., Sivan R., Kandelis Y., Spira G., Vlodavsky I., Keshet E., Neufeld G. VEGF145, a secreted vascular endothelial growth factor isoform that binds to extracellular matrix. J Biol Chem. 1997 Mar 14;272(11):7151–7158. doi: 10.1074/jbc.272.11.7151. [DOI] [PubMed] [Google Scholar]
- Quinn T. P., Peters K. G., De Vries C., Ferrara N., Williams L. T. Fetal liver kinase 1 is a receptor for vascular endothelial growth factor and is selectively expressed in vascular endothelium. Proc Natl Acad Sci U S A. 1993 Aug 15;90(16):7533–7537. doi: 10.1073/pnas.90.16.7533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salceda S., Caro J. Hypoxia-inducible factor 1alpha (HIF-1alpha) protein is rapidly degraded by the ubiquitin-proteasome system under normoxic conditions. Its stabilization by hypoxia depends on redox-induced changes. J Biol Chem. 1997 Sep 5;272(36):22642–22647. doi: 10.1074/jbc.272.36.22642. [DOI] [PubMed] [Google Scholar]
- Shweiki D., Itin A., Soffer D., Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992 Oct 29;359(6398):843–845. doi: 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- Simonetti R. G., Cammà C., Fiorello F., Politi F., D'Amico G., Pagliaro L. Hepatocellular carcinoma. A worldwide problem and the major risk factors. Dig Dis Sci. 1991 Jul;36(7):962–972. doi: 10.1007/BF01297149. [DOI] [PubMed] [Google Scholar]
- Stein I., Itin A., Einat P., Skaliter R., Grossman Z., Keshet E. Translation of vascular endothelial growth factor mRNA by internal ribosome entry: implications for translation under hypoxia. Mol Cell Biol. 1998 Jun;18(6):3112–3119. doi: 10.1128/mcb.18.6.3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K., Hayashi N., Miyamoto Y., Yamamoto M., Ohkawa K., Ito Y., Sasaki Y., Yamaguchi Y., Nakase H., Noda K. Expression of vascular permeability factor/vascular endothelial growth factor in human hepatocellular carcinoma. Cancer Res. 1996 Jul 1;56(13):3004–3009. [PubMed] [Google Scholar]
- Takahashi A., Sasaki H., Kim S. J., Tobisu K., Kakizoe T., Tsukamoto T., Kumamoto Y., Sugimura T., Terada M. Markedly increased amounts of messenger RNAs for vascular endothelial growth factor and placenta growth factor in renal cell carcinoma associated with angiogenesis. Cancer Res. 1994 Aug 1;54(15):4233–4237. [PubMed] [Google Scholar]
- Takahashi Y., Kitadai Y., Bucana C. D., Cleary K. R., Ellis L. M. Expression of vascular endothelial growth factor and its receptor, KDR, correlates with vascularity, metastasis, and proliferation of human colon cancer. Cancer Res. 1995 Sep 15;55(18):3964–3968. [PubMed] [Google Scholar]
- Terman B. I., Dougher-Vermazen M., Carrion M. E., Dimitrov D., Armellino D. C., Gospodarowicz D., Böhlen P. Identification of the KDR tyrosine kinase as a receptor for vascular endothelial cell growth factor. Biochem Biophys Res Commun. 1992 Sep 30;187(3):1579–1586. doi: 10.1016/0006-291x(92)90483-2. [DOI] [PubMed] [Google Scholar]
- Tischer E., Mitchell R., Hartman T., Silva M., Gospodarowicz D., Fiddes J. C., Abraham J. A. The human gene for vascular endothelial growth factor. Multiple protein forms are encoded through alternative exon splicing. J Biol Chem. 1991 Jun 25;266(18):11947–11954. [PubMed] [Google Scholar]
- Torimura T., Sata M., Ueno T., Kin M., Tsuji R., Suzaku K., Hashimoto O., Sugawara H., Tanikawa K. Increased expression of vascular endothelial growth factor is associated with tumor progression in hepatocellular carcinoma. Hum Pathol. 1998 Sep;29(9):986–991. doi: 10.1016/s0046-8177(98)90205-2. [DOI] [PubMed] [Google Scholar]
- Waltenberger J., Claesson-Welsh L., Siegbahn A., Shibuya M., Heldin C. H. Different signal transduction properties of KDR and Flt1, two receptors for vascular endothelial growth factor. J Biol Chem. 1994 Oct 28;269(43):26988–26995. [PubMed] [Google Scholar]
- Wheeler-Jones C., Abu-Ghazaleh R., Cospedal R., Houliston R. A., Martin J., Zachary I. Vascular endothelial growth factor stimulates prostacyclin production and activation of cytosolic phospholipase A2 in endothelial cells via p42/p44 mitogen-activated protein kinase. FEBS Lett. 1997 Dec 22;420(1):28–32. doi: 10.1016/s0014-5793(97)01481-6. [DOI] [PubMed] [Google Scholar]
- Yamaguchi R., Yano H., Iemura A., Ogasawara S., Haramaki M., Kojiro M. Expression of vascular endothelial growth factor in human hepatocellular carcinoma. Hepatology. 1998 Jul;28(1):68–77. doi: 10.1002/hep.510280111. [DOI] [PubMed] [Google Scholar]
- Yoshiji H., Kuriyama S., Hicklin D. J., Huber J., Yoshii J., Miyamoto Y., Kawata M., Ikenaka Y., Nakatani T., Tsujinoue H. KDR/Flk-1 is a major regulator of vascular endothelial growth factor-induced tumor development and angiogenesis in murine hepatocellular carcinoma cells. Hepatology. 1999 Nov;30(5):1179–1186. doi: 10.1002/hep.510300509. [DOI] [PubMed] [Google Scholar]
- de Vries C., Escobedo J. A., Ueno H., Houck K., Ferrara N., Williams L. T. The fms-like tyrosine kinase, a receptor for vascular endothelial growth factor. Science. 1992 Feb 21;255(5047):989–991. doi: 10.1126/science.1312256. [DOI] [PubMed] [Google Scholar]