Abstract
BACKGROUND—5-Fluorouracil (FU) in association with folinic acid (FA) is the most frequently used chemotherapeutic agent in colorectal cancer but it often causes diarrhoea. Animal and human studies suggest that glutamine stimulates intestinal mucosal growth. AIM—To determine if oral glutamine prevents changes in intestinal absorption (IA) and permeability (IP) induced by FU/FA. METHODS—Seventy chemotherapy naive patients with colorectal cancer were randomly assigned to oral glutamine (18 g/day) or placebo before the first cycle of FU (450 mg/m2) and FA (100 mg/m2) administered intravenously for five days. Treatment was continued for 15 days, starting five days before the beginning of chemotherapy. IA (D-xylose urinary excretion) and IP (cellobiose-mannitol test) were assessed at baseline and four and five days after the end of the first cycle of chemotherapy, respectively. Patients kept a daily record of diarrhoea, scored using the classification system of the National Cancer Institute (Bethesda, Maryland, USA). Duration of diarrhoea was recorded and the area under the curve (AUC) was calculated for each patient. RESULTS—Baseline patient characteristics and basal values of IP and IA tests were similar in the two arms. After one cycle of chemotherapy, the reduction in IA (D-xylose absorption) was more marked in the placebo arm (7.1% v 3.8%; p=0.02); reduction of IP to mannitol was higher in the placebo arm (9.2% v 4.5%; p=0.02); and urinary recovery of cellobiose was not different between the study arms (p=0.60). Accordingly, the cellobiose-mannitol ratio increased more in the placebo arm (0.037 v 0.012; p=0.04). Average AUC of diarrhoea (1.9 v 4.5; p=0.09) and average number of loperamide tablets taken (0.4 v 2.6; p=0.002) were reduced in the glutamine arm. CONCLUSIONS—Glutamine reduces changes in IA and IP induced by FU and may have a protective effect on FU induced diarrhoea. Keywords: glutamine; 5-fluorouracil; colorectal cancer; randomised trial
Full Text
The Full Text of this article is available as a PDF (145.5 KB).
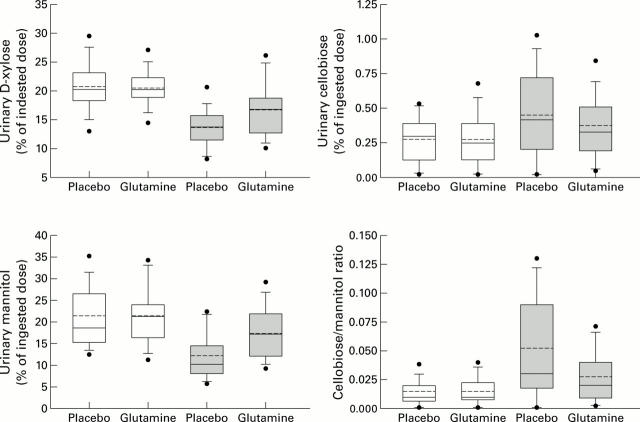
Figure 1 .
Box plots of pre- (open boxes) and post- (shaded boxes) treatment values for intestinal absorption and intestinal permeability parameters. Solid lines indicate the 5th, 25th, 50th, 75th, and 95th percentiles; broken lines indicate mean values; solid dots indicate upper and lower values.
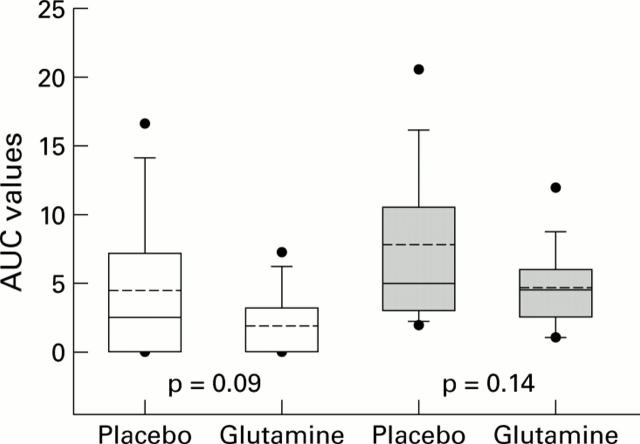
Figure 2 .
Box plots of area under the curve (AUC) values by treatment arm for all patients (open boxes) and for those with diarrhoea (shaded boxes). Solid lines indicate the 5th, 25th, 50th, 75th, and 95th percentiles; broken lines indicate mean values; solid dots indicate upper and lower values.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P. M., Schroeder G., Skubitz K. M. Oral glutamine reduces the duration and severity of stomatitis after cytotoxic cancer chemotherapy. Cancer. 1998 Oct 1;83(7):1433–1439. doi: 10.1002/(sici)1097-0142(19981001)83:7<1433::aid-cncr22>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Baskerville A., Batter-Hatton D. Intestinal lesions induced experimentally by methotrexate. Br J Exp Pathol. 1977 Dec;58(6):663–669. [PMC free article] [PubMed] [Google Scholar]
- Baskerville A., Hambleton P., Benbough J. E. Pathological features of glutaminase toxicity. Br J Exp Pathol. 1980 Apr;61(2):132–138. [PMC free article] [PubMed] [Google Scholar]
- Bjarnason I., MacPherson A., Hollander D. Intestinal permeability: an overview. Gastroenterology. 1995 May;108(5):1566–1581. doi: 10.1016/0016-5085(95)90708-4. [DOI] [PubMed] [Google Scholar]
- Craig R. M., Atkinson A. J., Jr D-xylose testing: a review. Gastroenterology. 1988 Jul;95(1):223–231. doi: 10.1016/0016-5085(88)90318-6. [DOI] [PubMed] [Google Scholar]
- Fox A. D., Kripke S. A., De Paula J., Berman J. M., Settle R. G., Rombeau J. L. Effect of a glutamine-supplemented enteral diet on methotrexate-induced enterocolitis. JPEN J Parenter Enteral Nutr. 1988 Jul-Aug;12(4):325–331. doi: 10.1177/0148607188012004325. [DOI] [PubMed] [Google Scholar]
- Francini G., Petrioli R., Lorenzini L., Mancini S., Armenio S., Tanzini G., Marsili S., Aquino A., Marzocca G., Civitelli S. Folinic acid and 5-fluorouracil as adjuvant chemotherapy in colon cancer. Gastroenterology. 1994 Apr;106(4):899–906. doi: 10.1016/0016-5085(94)90748-x. [DOI] [PubMed] [Google Scholar]
- Gardner M. L., Samson R. R., Heading R. C. Changes in absorptive and peptide hydrolase activities in rat small intestine after administration of 5-fluorouracil. Clin Sci Mol Med. 1978 Apr;54(4):411–418. doi: 10.1042/cs0540411. [DOI] [PubMed] [Google Scholar]
- Jebb S. A., Osborne R. J., Maughan T. S., Mohideen N., Mack P., Mort D., Shelley M. D., Elia M. 5-fluorouracil and folinic acid-induced mucositis: no effect of oral glutamine supplementation. Br J Cancer. 1994 Oct;70(4):732–735. doi: 10.1038/bjc.1994.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juby L. D., Rothwell J., Axon A. T. Cellobiose/mannitol sugar test--a sensitive tubeless test for coeliac disease: results on 1010 unselected patients. Gut. 1989 Apr;30(4):476–480. doi: 10.1136/gut.30.4.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan K., Elia M. Factors affecting the stability of L-glutamine in solution. Clin Nutr. 1991 Aug;10(4):186–192. doi: 10.1016/0261-5614(91)90037-d. [DOI] [PubMed] [Google Scholar]
- Khan K., Hardy G., McElroy B., Elia M. The stability of L-glutamine in total parenteral nutrition solutions. Clin Nutr. 1991 Aug;10(4):193–198. doi: 10.1016/0261-5614(91)90038-e. [DOI] [PubMed] [Google Scholar]
- Klimberg V. S., Souba W. W., Dolson D. J., Salloum R. M., Hautamaki R. D., Plumley D. A., Mendenhall W. M., Bova F. J., Khan S. R., Hackett R. L. Prophylactic glutamine protects the intestinal mucosa from radiation injury. Cancer. 1990 Jul 1;66(1):62–68. doi: 10.1002/1097-0142(19900701)66:1<62::aid-cncr2820660113>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Kubota A., Meguid M. M., Hitch D. C. Amino acid profiles correlate diagnostically with organ site in three kinds of malignant tumors. Cancer. 1992 May 1;69(9):2343–2348. doi: 10.1002/1097-0142(19920501)69:9<2343::aid-cncr2820690924>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Labianca R., Pancera G., Luporini G. Factors influencing response rates for advanced colorectal cancer chemotherapy. Ann Oncol. 1996 Nov;7(9):901–906. doi: 10.1093/oxfordjournals.annonc.a010791. [DOI] [PubMed] [Google Scholar]
- Leichman C. G., Fleming T. R., Muggia F. M., Tangen C. M., Ardalan B., Doroshow J. H., Meyers F. J., Holcombe R. F., Weiss G. R., Mangalik A. Phase II study of fluorouracil and its modulation in advanced colorectal cancer: a Southwest Oncology Group study. J Clin Oncol. 1995 Jun;13(6):1303–1311. doi: 10.1200/JCO.1995.13.6.1303. [DOI] [PubMed] [Google Scholar]
- Matthews J. N., Altman D. G., Campbell M. J., Royston P. Analysis of serial measurements in medical research. BMJ. 1990 Jan 27;300(6719):230–235. doi: 10.1136/bmj.300.6719.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olaison G., Sjödahl R., Tagesson C. Abnormal intestinal permeability in Crohn's disease. A possible pathogenic factor. Scand J Gastroenterol. 1990 Apr;25(4):321–328. doi: 10.3109/00365529009095493. [DOI] [PubMed] [Google Scholar]
- Peled Y., Doron O., Laufer H., Bujanover Y., Gilat T. D-xylose absorption test. Urine or blood? Dig Dis Sci. 1991 Feb;36(2):188–192. doi: 10.1007/BF01300755. [DOI] [PubMed] [Google Scholar]
- Petrelli N., Herrera L., Rustum Y., Burke P., Creaven P., Stulc J., Emrich L. J., Mittelman A. A prospective randomized trial of 5-fluorouracil versus 5-fluorouracil and high-dose leucovorin versus 5-fluorouracil and methotrexate in previously untreated patients with advanced colorectal carcinoma. J Clin Oncol. 1987 Oct;5(10):1559–1565. doi: 10.1200/JCO.1987.5.10.1559. [DOI] [PubMed] [Google Scholar]
- Poon M. A., O'Connell M. J., Moertel C. G., Wieand H. S., Cullinan S. A., Everson L. K., Krook J. E., Mailliard J. A., Laurie J. A., Tschetter L. K. Biochemical modulation of fluorouracil: evidence of significant improvement of survival and quality of life in patients with advanced colorectal carcinoma. J Clin Oncol. 1989 Oct;7(10):1407–1418. doi: 10.1200/JCO.1989.7.10.1407. [DOI] [PubMed] [Google Scholar]
- Powell-Tuck J., Jamieson C. P., Bettany G. E., Obeid O., Fawcett H. V., Archer C., Murphy D. L. A double blind, randomised, controlled trial of glutamine supplementation in parenteral nutrition. Gut. 1999 Jul;45(1):82–88. doi: 10.1136/gut.45.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolston D. D., Mathan V. I. Xylose transport in the human jejunum. Dig Dis Sci. 1989 Apr;34(4):553–558. doi: 10.1007/BF01536332. [DOI] [PubMed] [Google Scholar]
- Siber G. R., Mayer R. J., Levin M. J. Increased gastrointestinal absorption of large molecules in patients after 5-fluorouracil therapy for metastatic colon carcinoma. Cancer Res. 1980 Oct;40(10):3430–3436. [PubMed] [Google Scholar]
- Siber G. R., Mayer R. J., Levin M. J. Increased gastrointestinal absorption of large molecules in patients after 5-fluorouracil therapy for metastatic colon carcinoma. Cancer Res. 1980 Oct;40(10):3430–3436. [PubMed] [Google Scholar]
- Slavin R. E., Dias M. A., Saral R. Cytosine arabinoside induced gastrointestinal toxic alterations in sequential chemotherapeutic protocols: a clinical-pathologic study of 33 patients. Cancer. 1978 Oct;42(4):1747–1759. doi: 10.1002/1097-0142(197810)42:4<1747::aid-cncr2820420413>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Souba W. W. Glutamine: a key substrate for the splanchnic bed. Annu Rev Nutr. 1991;11:285–308. doi: 10.1146/annurev.nu.11.070191.001441. [DOI] [PubMed] [Google Scholar]
- Strobel S., Brydon W. G., Ferguson A. Cellobiose/mannitol sugar permeability test complements biopsy histopathology in clinical investigation of the jejunum. Gut. 1984 Nov;25(11):1241–1246. doi: 10.1136/gut.25.11.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremel H., Kienle B., Weilemann L. S., Stehle P., Fürst P. Glutamine dipeptide-supplemented parenteral nutrition maintains intestinal function in the critically ill. Gastroenterology. 1994 Dec;107(6):1595–1601. doi: 10.1016/0016-5085(94)90797-8. [DOI] [PubMed] [Google Scholar]
- Wilmore D. W. Glutamine and the gut. Gastroenterology. 1994 Dec;107(6):1885–1886. doi: 10.1016/0016-5085(94)90836-2. [DOI] [PubMed] [Google Scholar]
- Windmueller H. G., Spaeth A. E. Identification of ketone bodies and glutamine as the major respiratory fuels in vivo for postabsorptive rat small intestine. J Biol Chem. 1978 Jan 10;253(1):69–76. [PubMed] [Google Scholar]
- Windmueller H. G., Spaeth A. E. Respiratory fuels and nitrogen metabolism in vivo in small intestine of fed rats. Quantitative importance of glutamine, glutamate, and aspartate. J Biol Chem. 1980 Jan 10;255(1):107–112. [PubMed] [Google Scholar]
- Windmueller H. G., Spaeth A. E. Uptake and metabolism of plasma glutamine by the small intestine. J Biol Chem. 1974 Aug 25;249(16):5070–5079. [PubMed] [Google Scholar]
- de Roy van Zuidewijn D. B., Schillings P. H., Wobbes T., Hendriks T., de Boer H. H. Morphometric analysis of the effects of antineoplastic drugs on mucosa of normal ileum and ileal anastomoses in rats. Exp Mol Pathol. 1992 Apr;56(2):96–107. doi: 10.1016/0014-4800(92)90027-9. [DOI] [PubMed] [Google Scholar]
- van der Hulst R. R., van Kreel B. K., von Meyenfeldt M. F., Brummer R. J., Arends J. W., Deutz N. E., Soeters P. B. Glutamine and the preservation of gut integrity. Lancet. 1993 May 29;341(8857):1363–1365. doi: 10.1016/0140-6736(93)90939-e. [DOI] [PubMed] [Google Scholar]