Abstract
BACKGROUND—Multiorgan failure is a severe life threatening state where present therapeutic approaches are suboptimal. Epidermal growth factor (EGF) is a potent stimulant of repair in in vitro and in vivo models. We therefore examined its potential beneficial effect in reducing mortality and injury induced by the noxious agent thioacetamide (TAA). METHODS—Mice (20 per group) were fasted overnight and received a single intraperitoneal dose of human recombinant EGF at 10 or 30 µg/kg or saline (control). Either 30 minutes before or after EGF, all animals also received TAA (40 mg/kg intraperitoneally). Twenty four hours later, surviving animals were killed, tissues collected, and degree of organ injury assessed. RESULTS—Fifty per cent (10/20) of control animals died within the first 24 hour period. Mortality was almost completely prevented by the higher dose of EGF whether given before or after TAA (p<0.01) and was reduced by about 50% with the lower dose of EGF. In control animals, the entire length of the jejunum and ileum had necrosis with or without mucosal denudation. In contrast, necrosis affected only about 10-20% of the total length in EGF treated groups (both p<0.01 v control). Control animals showed marked glomerular tuft collapse, interstitial haemorrhage, and increased plasma creatinine levels. These effects were significantly reduced in animals given EGF (30 µg/kg; p<0.01). All groups showed similar changes in liver histology (centrilobular necrosis) and alanine transaminase levels (10-fold increase). CONCLUSIONS—Although EGF did not prevent the hepatotoxicity associated with TAA, it reduced mortality, renal injury, and gastrointestinal damage. These studies provide preliminary evidence that EGF may be a novel approach for the prevention and/or treatment of multiorgan failure. Keywords: gastrointestinal damage; nephrotoxicity; liver injury
Full Text
The Full Text of this article is available as a PDF (260.3 KB).
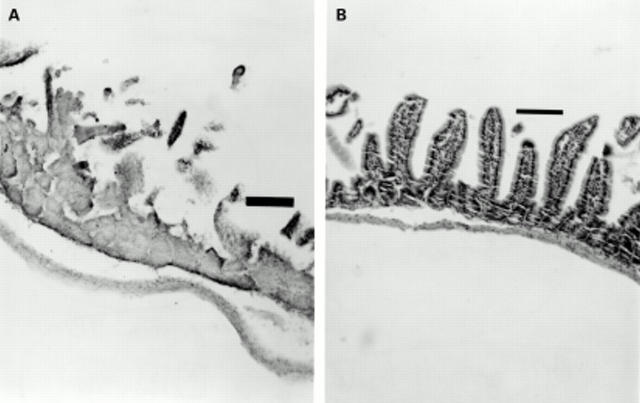
Figure 1 .
Thioacetamide (TAA) induced small intestinal injury. (A) Twenty four hours after administration of TAA (40 mg/kg) to mice, the normal villus structure of the jejunum and ileum is lost and marked necrosis is seen within the remaining mucosa. (B) Administration of epidermal growth factor (EGF, 30 µg/kg), 30 minutes prior to TAA, markedly preserved small intestinal architecture. Similar preservation was seen when a dose of 10 µg/kg of EGF was used (not shown). Tissues were stained with haematoxylin and eosin. Original magnification ×10. Bar=100 µm.
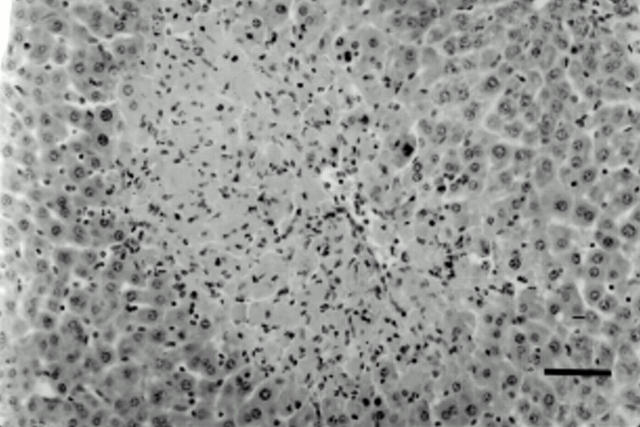
Figure 2 .
Thioacetamide (TAA) induced hepatic injury. TAA treated animals showed marked hepatic injury, mainly centrilobular necrosis with occasional haemorrhagic foci. The histological appearance was similar in all treatment groups. Tissues were stained with haematoxylin and eosin. Original magnification ×20. Bar=50 µm.
Figure 3 .
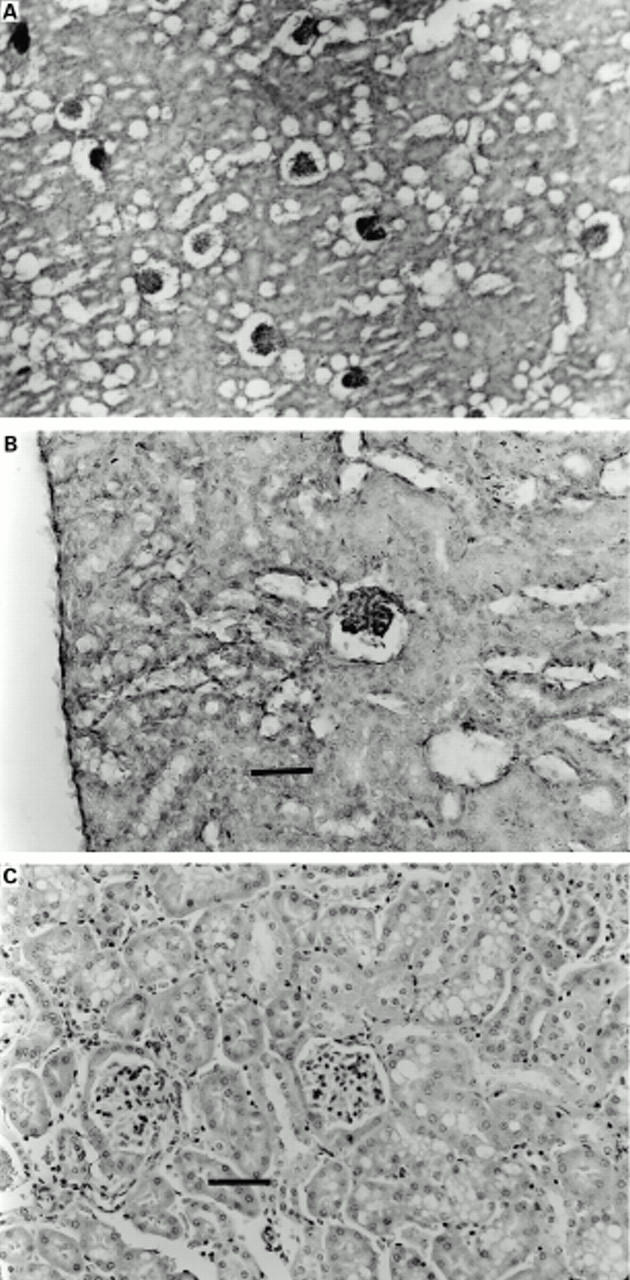
Thioacetamide (TAA) induced renal injury. Tissues were stained with haematoxylin and eosin. The renal tissue of TAA treated animals showed extensive collapse of the glomerular tufts.. Original magnification ×10. (B) Higher power photomicrograph of the same tissue demonstrating that only minor degenerative acute changes in the epithelial cells of the tubular system were seen. Original magnification ×20. Bar=50 µm. (C) Pretreatment with EGF reduced the renal changes caused by TAA. Original magnification ×20. Bar=50 µm.
Figure 4 .
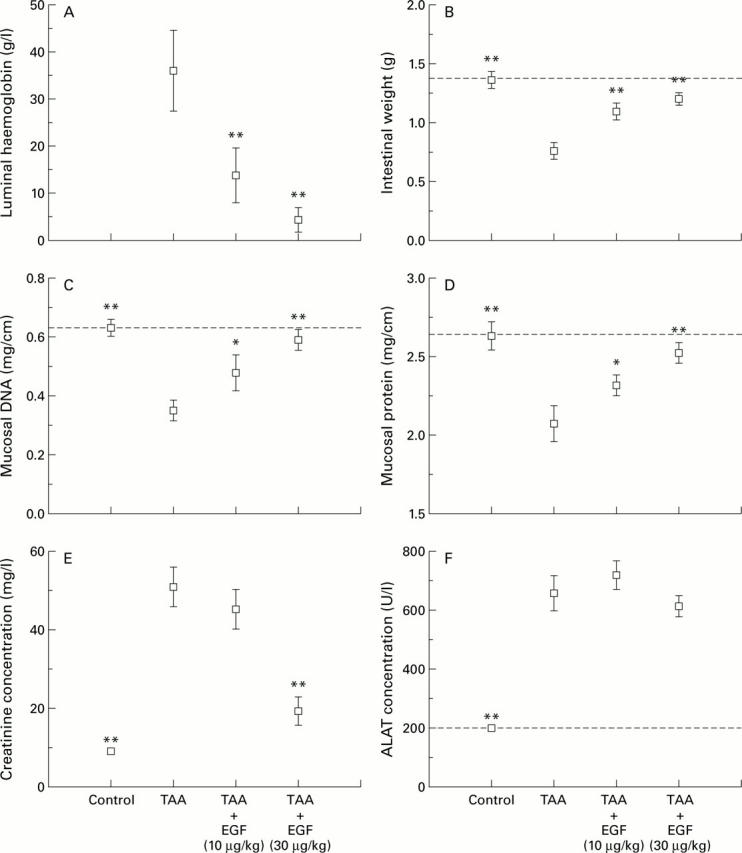
Effect of epidermal growth factor (EGF) given 30 minutes after thioacetamide (TAA) on multiple organ systems. EGF reduced luminal bleeding (A), and the reduction in wet weight (B), DNA (C), and protein content (D) of the gut caused by TAA. The rise in plasma creatinine caused by TAA was also reduced by administration of EGF (E) although it did not influence the rise in serum alanine aminotransferase (ALAT) (F). *p<0.05, **p<0.01 compared with animals given TAA alone.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barker E. A., Smuckler E. A. Nonhepatic thioacetamide injury. II. The morphologic features of proximal renal tubular injury. Am J Pathol. 1974 Mar;74(3):575–590. [PMC free article] [PubMed] [Google Scholar]
- Berlanga J., Caballero M. E., Ramirez D., Torres A., Valenzuela C., Lodos J., Playford R. J. Epidermal growth factor protects against carbon tetrachloride-induced hepatic injury. Clin Sci (Lond) 1998 Mar;94(3):219–223. doi: 10.1042/cs0940219. [DOI] [PubMed] [Google Scholar]
- Conteas C. N., Majumdar A. P. The effects of gastrin, epidermal growth factor, and somatostatin on DNA synthesis in a small intestinal crypt cell line (IEC-6). Proc Soc Exp Biol Med. 1987 Mar;184(3):307–311. doi: 10.3181/00379727-184-42484. [DOI] [PubMed] [Google Scholar]
- Dyroff M. C., Neal R. A. Identification of the major protein adduct formed in rat liver after thioacetamide administration. Cancer Res. 1981 Sep;41(9 Pt 1):3430–3435. [PubMed] [Google Scholar]
- Gan B. S., Hollenberg M. D., MacCannell K. L., Lederis K., Winkler M. E., Derynck R. Distinct vascular actions of epidermal growth factor-urogastrone and transforming growth factor-alpha. J Pharmacol Exp Ther. 1987 Jul;242(1):331–337. [PubMed] [Google Scholar]
- Goodlad R. A., Lee C. Y., Wright N. A. Cell proliferation in the small intestine and colon of intravenously fed rats: effects of urogastrone-epidermal growth factor. Cell Prolif. 1992 Sep;25(5):393–404. doi: 10.1111/j.1365-2184.1992.tb01449.x. [DOI] [PubMed] [Google Scholar]
- Jansen M. J., Hendriks T., Verhofstad A. A., Lange W., Geeraedts L. M., Jr, Goris R. J. Gradual development of organ damage in the murine zymosan-induced multiple organ dysfunction syndrome. Shock. 1997 Oct;8(4):261–267. doi: 10.1097/00024382-199710000-00005. [DOI] [PubMed] [Google Scholar]
- Logan S. K., Falasca M., Hu P., Schlessinger J. Phosphatidylinositol 3-kinase mediates epidermal growth factor-induced activation of the c-Jun N-terminal kinase signaling pathway. Mol Cell Biol. 1997 Oct;17(10):5784–5790. doi: 10.1128/mcb.17.10.5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesa M. L., Carrizosa R., Martínez-Honduvilla C., Benito M., Fabregat I. Changes in rat liver gene expression induced by thioacetamide: protective role of S-adenosyl-L-methionine by a glutathione-dependent mechanism. Hepatology. 1996 Mar;23(3):600–606. doi: 10.1002/hep.510230327. [DOI] [PubMed] [Google Scholar]
- Nguyen T. T., Gilpin D. A., Meyer N. A., Herndon D. N. Current treatment of severely burned patients. Ann Surg. 1996 Jan;223(1):14–25. doi: 10.1097/00000658-199601000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega M. A., Torres M. I., Fernández M. I., Rios A., Sánchez-Pozo A., Gil A. Hepatotoxic agent thioacetamide induces biochemical and histological alterations in rat small intestine. Dig Dis Sci. 1997 Aug;42(8):1715–1723. doi: 10.1023/a:1018817600238. [DOI] [PubMed] [Google Scholar]
- Parks D. A., Bulkley G. B., Granger D. N., Hamilton S. R., McCord J. M. Ischemic injury in the cat small intestine: role of superoxide radicals. Gastroenterology. 1982 Jan;82(1):9–15. [PubMed] [Google Scholar]
- Peeling J., Shoemaker L., Gauthier T., Benarroch A., Sutherland G. R., Minuk G. Y. Cerebral metabolic and histological effects of thioacetamide-induced liver failure. Am J Physiol. 1993 Sep;265(3 Pt 1):G572–G578. doi: 10.1152/ajpgi.1993.265.3.G572. [DOI] [PubMed] [Google Scholar]
- Playford R. J., Marchbank T., Calnan D. P., Calam J., Royston P., Batten J. J., Hansen H. F. Epidermal growth factor is digested to smaller, less active forms in acidic gastric juice. Gastroenterology. 1995 Jan;108(1):92–101. doi: 10.1016/0016-5085(95)90012-8. [DOI] [PubMed] [Google Scholar]
- Price L. T., Chen Y., Frank L. Epidermal growth factor increases antioxidant enzyme and surfactant system development during hyperoxia and protects fetal rat lungs in vitro from hyperoxic toxicity. Pediatr Res. 1993 Nov;34(5):577–585. doi: 10.1203/00006450-199311000-00005. [DOI] [PubMed] [Google Scholar]
- Procaccino F., Reinshagen M., Hoffmann P., Zeeh J. M., Lakshmanan J., McRoberts J. A., Patel A., French S., Eysselein V. E. Protective effect of epidermal growth factor in an experimental model of colitis in rats. Gastroenterology. 1994 Jul;107(1):12–17. doi: 10.1016/0016-5085(94)90055-8. [DOI] [PubMed] [Google Scholar]
- REITMAN S., FRANKEL S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol. 1957 Jul;28(1):56–63. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- Sarosiek J., Bilski J., Murty V. L., Slomiany A., Slomiany B. L. Role of salivary epidermal growth factor in the maintenance of physicochemical characteristics of oral and gastric mucosal mucus coat. Biochem Biophys Res Commun. 1988 May 16;152(3):1421–1427. doi: 10.1016/s0006-291x(88)80444-3. [DOI] [PubMed] [Google Scholar]
- Tepperman B. L., Soper B. D. Effect of epidermal growth factor, transforming growth factor alpha and nerve growth factor on gastric mucosal integrity and microcirculation in the rat. Regul Pept. 1994 Feb 3;50(1):13–21. doi: 10.1016/0167-0115(94)90186-4. [DOI] [PubMed] [Google Scholar]
- Uribe J. M., Barrett K. E. Nonmitogenic actions of growth factors: an integrated view of their role in intestinal physiology and pathophysiology. Gastroenterology. 1997 Jan;112(1):255–268. [PubMed] [Google Scholar]
- Witzmann F. A., Fultz C. D., Mangipudy R. S., Mehendale H. M. Two-dimensional electrophoretic analysis of compartment-specific hepatic protein charge modification induced by thioacetamide exposure in rats. Fundam Appl Toxicol. 1996 May;31(1):124–132. doi: 10.1006/faat.1996.0083. [DOI] [PubMed] [Google Scholar]
- Zimmerman J. E., Knaus W. A., Sun X., Wagner D. P. Severity stratification and outcome prediction for multisystem organ failure and dysfunction. World J Surg. 1996 May;20(4):401–405. doi: 10.1007/s002689900063. [DOI] [PubMed] [Google Scholar]
- Zimmermann T., Franke H., Dargel R. Biochemical and substructural studies on hepatic and serum lipoprotein metabolism after acute liver injury induced by thioacetamide in rats. Exp Pathol. 1985;28(4):225–233. doi: 10.1016/s0232-1513(85)80012-8. [DOI] [PubMed] [Google Scholar]
- Zimmermann T., Müller A., Machnik G., Franke H., Schubert H., Dargel R. Biochemical and morphological studies on production and regression of experimental liver cirrhosis induced by thioacetamide in Uje: WIST rats. Z Versuchstierkd. 1987;30(5-6):165–180. [PubMed] [Google Scholar]