Abstract
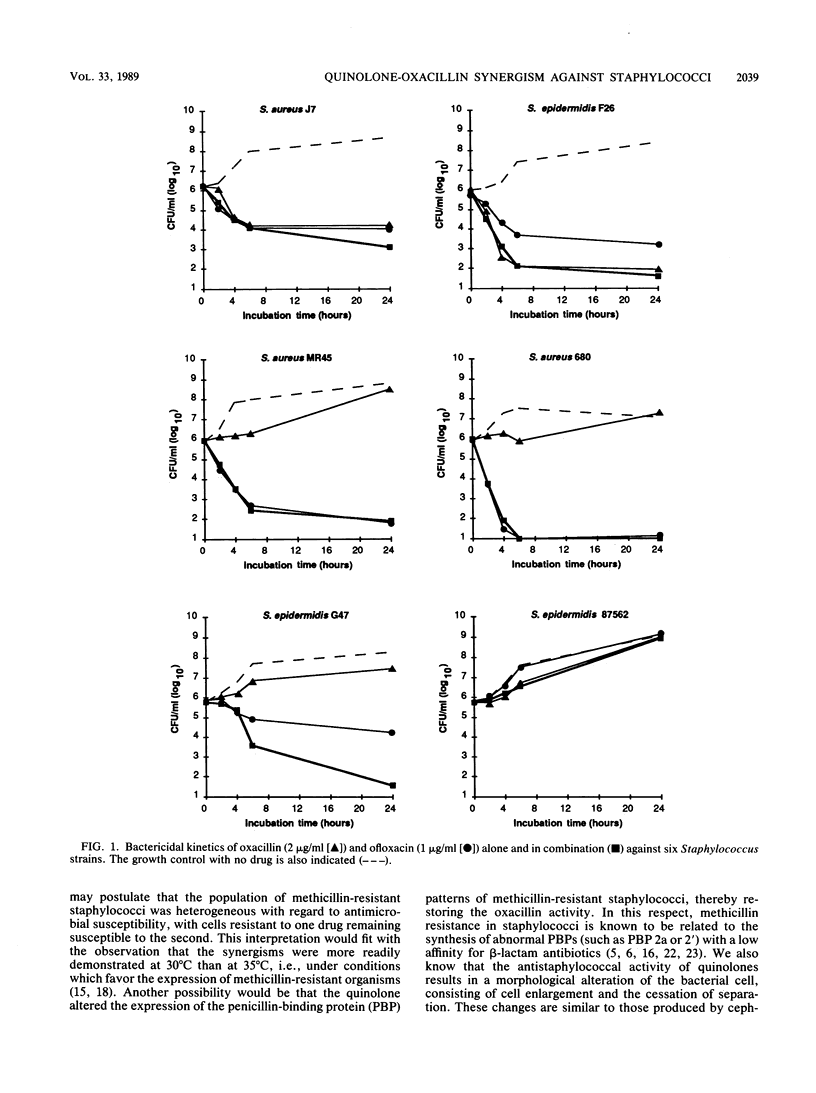
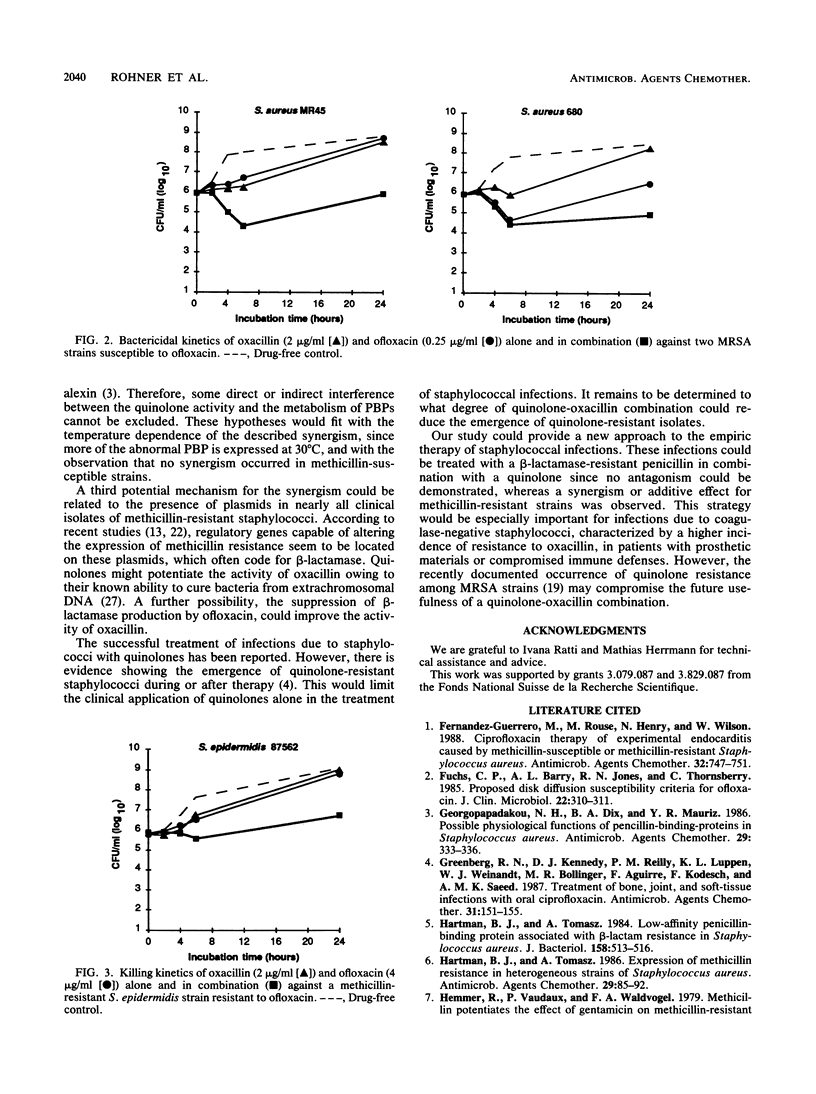
Various combinations of antistaphylococcal antimicrobial agents have been tested against 17 selected Staphylococcus isolates, including methicillin-susceptible and methicillin-resistant strains of S. aureus and coagulase-negative Staphylococcus species. With the checkerboard technique the following combinations were tested: oxacillin-ofloxacin, oxacillin-temafloxacin, oxacillin-fleroxacin, vancomycin-fleroxacin, gentamicin-fleroxacin, and rifampin-fleroxacin. Against methicillin-resistant staphylococci the combination oxacillin-quinolone tested at 35 degrees C always showed a fractional inhibitory concentration (FIC) index of less than 0.75, which is interpreted as synergistic or additive. Equal or more synergistic effects were observed at 30 degrees C. In contrast, when methicillin-susceptible Staphylococcus species were tested, the FIC for the combination oxacillin-quinolone was always 1 or 2, which is considered to be indifferent. For the other mentioned combinations the FICs were also 1 or 2. Killing kinetics showed synergistic or additive bactericidal activity for the combination oxacillin-ofloxacin against methicillin-resistant Staphylococcus species, killing 1.5 to 2.8 log10 CFU more of these per ml than did the most active drug after 24 h of incubation. This difference was not observed for methicillin-susceptible strains. In vitro evidence for the potential clinical use of quinolones in treating infections due to methicillin-resistant staphylococci in combination with a beta-lactamase-resistant penicillin is provided.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fernandez-Guerrero M., Rouse M., Henry N., Wilson W. Ciprofloxacin therapy of experimental endocarditis caused by methicillin-susceptible or methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1988 May;32(5):747–751. doi: 10.1128/aac.32.5.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs P. C., Barry A. L., Jones R. N., Thornsberry C. Proposed disk diffusion susceptibility criteria for ofloxacin. J Clin Microbiol. 1985 Aug;22(2):310–311. doi: 10.1128/jcm.22.2.310-311.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopapadakou N. H., Dix B. A., Mauriz Y. R. Possible physiological functions of penicillin-binding proteins in Staphylococcus aureus. Antimicrob Agents Chemother. 1986 Feb;29(2):333–336. doi: 10.1128/aac.29.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg R. N., Kennedy D. J., Reilly P. M., Luppen K. L., Weinandt W. J., Bollinger M. R., Aguirre F., Kodesch F., Saeed A. M. Treatment of bone, joint, and soft-tissue infections with oral ciprofloxacin. Antimicrob Agents Chemother. 1987 Feb;31(2):151–155. doi: 10.1128/aac.31.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman B. J., Tomasz A. Expression of methicillin resistance in heterogeneous strains of Staphylococcus aureus. Antimicrob Agents Chemother. 1986 Jan;29(1):85–92. doi: 10.1128/aac.29.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman B. J., Tomasz A. Low-affinity penicillin-binding protein associated with beta-lactam resistance in Staphylococcus aureus. J Bacteriol. 1984 May;158(2):513–516. doi: 10.1128/jb.158.2.513-516.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann M., Vaudaux P. E., Pittet D., Auckenthaler R., Lew P. D., Schumacher-Perdreau F., Peters G., Waldvogel F. A. Fibronectin, fibrinogen, and laminin act as mediators of adherence of clinical staphylococcal isolates to foreign material. J Infect Dis. 1988 Oct;158(4):693–701. doi: 10.1093/infdis/158.4.693. [DOI] [PubMed] [Google Scholar]
- McDougal L. K., Thornsberry C. The role of beta-lactamase in staphylococcal resistance to penicillinase-resistant penicillins and cephalosporins. J Clin Microbiol. 1986 May;23(5):832–839. doi: 10.1128/jcm.23.5.832-839.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opal S. M., Boyce J. M., Medeiros A. A., Mayer K. H., Lyhte L. W. Modification of homogeneous resistance in a methicillin-resistant strain of Staphylococcus aureus by acquisition of a beta-lactamase encoding plasmid. J Antimicrob Chemother. 1989 Mar;23(3):315–325. doi: 10.1093/jac/23.3.315. [DOI] [PubMed] [Google Scholar]
- Pearson R. D., Steigbigel R. T., Davis H. T., Chapman S. W. Method of reliable determination of minimal lethal antibiotic concentrations. Antimicrob Agents Chemother. 1980 Nov;18(5):699–708. doi: 10.1128/aac.18.5.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds P. E., Brown D. F. Penicillin-binding proteins of beta-lactam-resistant strains of Staphylococcus aureus. Effect of growth conditions. FEBS Lett. 1985 Nov 11;192(1):28–32. doi: 10.1016/0014-5793(85)80036-3. [DOI] [PubMed] [Google Scholar]
- Rossi L., Tonin E., Cheng Y. R., Fontana R. Regulation of penicillin-binding protein activity: description of a methicillin-inducible penicillin-binding protein in Staphylococcus aureus. Antimicrob Agents Chemother. 1985 May;27(5):828–831. doi: 10.1128/aac.27.5.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Røder B. L., Gutschik E. In-vitro activity of ciprofloxacin combined with either fusidic acid or rifampicin against Staphylococcus aureus. J Antimicrob Chemother. 1989 Mar;23(3):347–352. doi: 10.1093/jac/23.3.347. [DOI] [PubMed] [Google Scholar]
- Shalit I., Berger S. A., Gorea A., Frimerman H. Widespread quinolone resistance among methicillin-resistant Staphylococcus aureus isolates in a general hospital. Antimicrob Agents Chemother. 1989 Apr;33(4):593–594. doi: 10.1128/aac.33.4.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. M., Eng R. H., Tecson-Tumang F. Ciprofloxacin therapy for methicillin-resistant Staphylococcus aureus infections or colonizations. Antimicrob Agents Chemother. 1989 Feb;33(2):181–184. doi: 10.1128/aac.33.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor P. C., Schoenknecht F. D., Sherris J. C., Linner E. C. Determination of minimum bactericidal concentrations of oxacillin for Staphylococcus aureus: influence and significance of technical factors. Antimicrob Agents Chemother. 1983 Jan;23(1):142–150. doi: 10.1128/aac.23.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubukata K., Yamashita N., Konno M. Occurrence of a beta-lactam-inducible penicillin-binding protein in methicillin-resistant staphylococci. Antimicrob Agents Chemother. 1985 May;27(5):851–857. doi: 10.1128/aac.27.5.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utsui Y., Yokota T. Role of an altered penicillin-binding protein in methicillin- and cephem-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1985 Sep;28(3):397–403. doi: 10.1128/aac.28.3.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Auwera P. Interaction of gentamicin, dibekacin, netilmicin and amikacin with various penicillins, cephalosporins, minocycline and new fluoro-quinolones against Enterobacteriaceae and Pseudomonas aeruginosa. J Antimicrob Chemother. 1985 Nov;16(5):581–587. doi: 10.1093/jac/16.5.581. [DOI] [PubMed] [Google Scholar]
- Vaudaux P., Waldvogel F. A. Methicillin-resistant strains of Staphylococcus aureus: relation between expression of resistance and phagocytosis by polymorphonuclear leukocytes. J Infect Dis. 1979 May;139(5):547–552. doi: 10.1093/infdis/139.5.547. [DOI] [PubMed] [Google Scholar]
- Weisser J., Wiedemann B. Elimination of plasmids by enoxacin and ofloxacin at near inhibitory concentrations. J Antimicrob Chemother. 1986 Nov;18(5):575–583. doi: 10.1093/jac/18.5.575. [DOI] [PubMed] [Google Scholar]
- Yourassowsky E., Van der Linden M. P., Crokaert F., Glupczynski Y. Effect of antibiotics carry-over on bacterial counting by 'spiral plating'. J Antimicrob Chemother. 1988 Jan;21(1):138–140. doi: 10.1093/jac/21.1.138. [DOI] [PubMed] [Google Scholar]


