Abstract
BACKGROUND AND AIMS—Intestinal epithelial cell derived antimicrobial peptides of the defensin family may play a major role in host defence against microorganisms. Our aims were to (i) isolate, characterise, and investigate the processing of human defensin 5 (HD-5) in normal Paneth cells and (ii) investigate expression of HD-5 in active inflammatory bowel disease (IBD). METHODS—Antiserum raised against chemically synthesised putative mature HD-5 was used for immunohistochemistry and purification of HD-5 from extracts of normal terminal ileal crypts. RESULTS—In normal and Crohn's disease terminal ileum, HD-5 immunoreactivity was seen in Paneth cells and in some villous epithelial cells. Normal colonic mucosa did not express HD-5 but HD-5 immunoreactivity was seen in cells in the colonic crypt region of many IBD samples. N-terminal amino acid sequence analysis of HD-5 purified from normal terminal ileal Paneth cells consistently showed the predicted sequence of the precursor form of the peptide. Following stimulation of isolated intact normal terminal ileal crypts, a truncated form of HD-5, with the N-terminal sequence GEDNQLAIS, was detected in the supernatant. CONCLUSIONS—(i) HD-5 is present only in the precursor form in normal terminal ileal Paneth cells and is processed to the mature form during and/or after secretion, (ii) some villous epithelial cells express HD-5, and (iii) HD-5 is expressed by metaplastic Paneth cells in the colon in IBD. Keywords: inflammatory bowel disease; human defensin 5; Paneth cells
Full Text
The Full Text of this article is available as a PDF (399.9 KB).
Figure 1 .
Predicted amino acid sequence of human defensin 5 (HD-5), based on the cDNA.18 Single letter amino acid code for prepro HD-5 is shown. The N-terminus of mature HD-5 is speculative. Conserved cysteines, characteristic of the α-defensin family, are shown in bold and amino acid residue numbering is shown in parentheses.
Figure 2 .
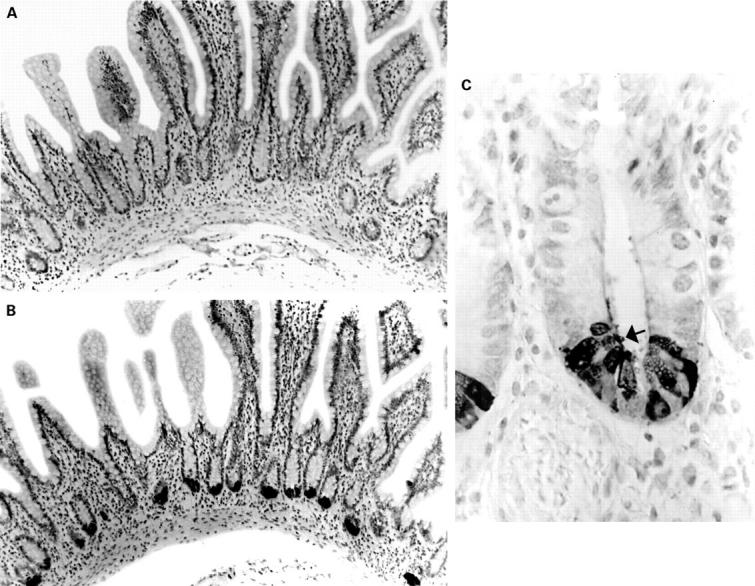
Normal terminal ileal mucosa stained with preimmune serum (A) and anti-human defensin 5 antisera (B, C). The antisera reacts strongly with Paneth cells, and immunoreactive material can be seen in the crypt lumen (C, arrow).
Figure 3 .
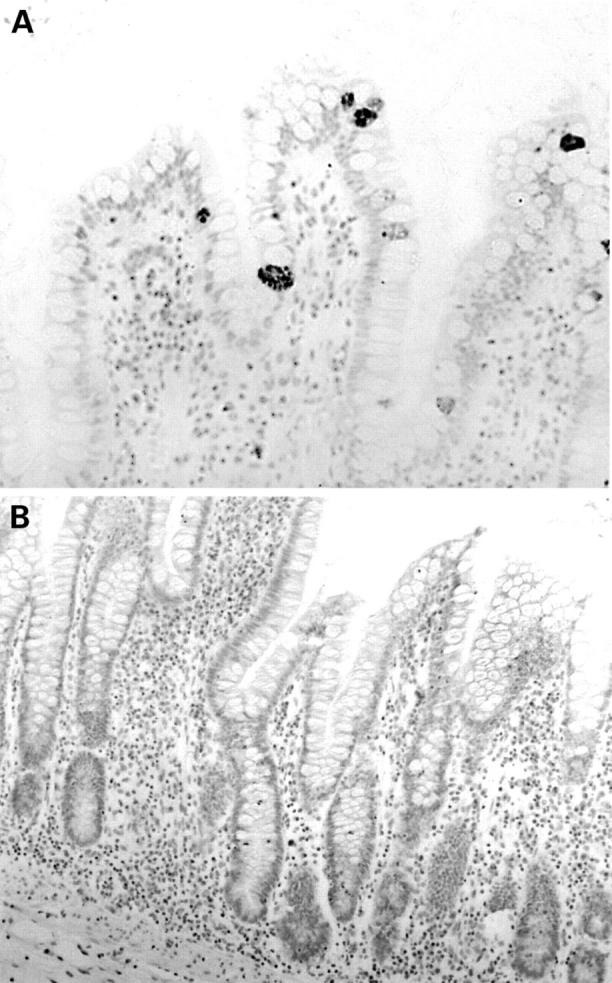
Normal terminal ileal mucosa (A) stained with anti-human defensin 5 (HD-5) antiserum showing occasional villous epithelial cells strongly immunoreactive for HD-5. (B) Section from the same specimen as (A) and stained with anti-HD-5 antiserum that had been preadsorbed with synthetic HD-5. Positive staining of HD-5 containing Paneth cells and villous epithelial cells is completely abolished.
Figure 4 .
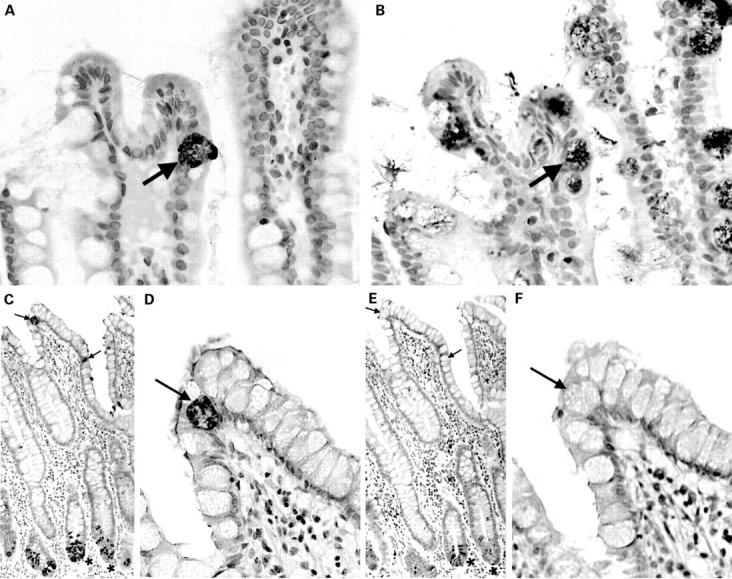
Sequential sections of normal terminal ileal mucosa stained with anti-human defensin 5 (HD-5) antiserum (A) and anti-intestinal trefoil factor (ITF) antiserum (B). The arrowed HD-5 immunoreactive villous epithelial cell also expresses ITF. A similar pair of sequential sections is stained with anti-HD-5 antiserum (C, D) and anti-lysozyme antiserum (E, F). As can be seen in the low power views (C, E), ileal Paneth cells express both HD-5 and lysozyme. (D, F) High power views of the same sections showing that the HD-5 immunoreactive villous epithelial cells (arrows) do not express lysozyme.
Figure 5 .
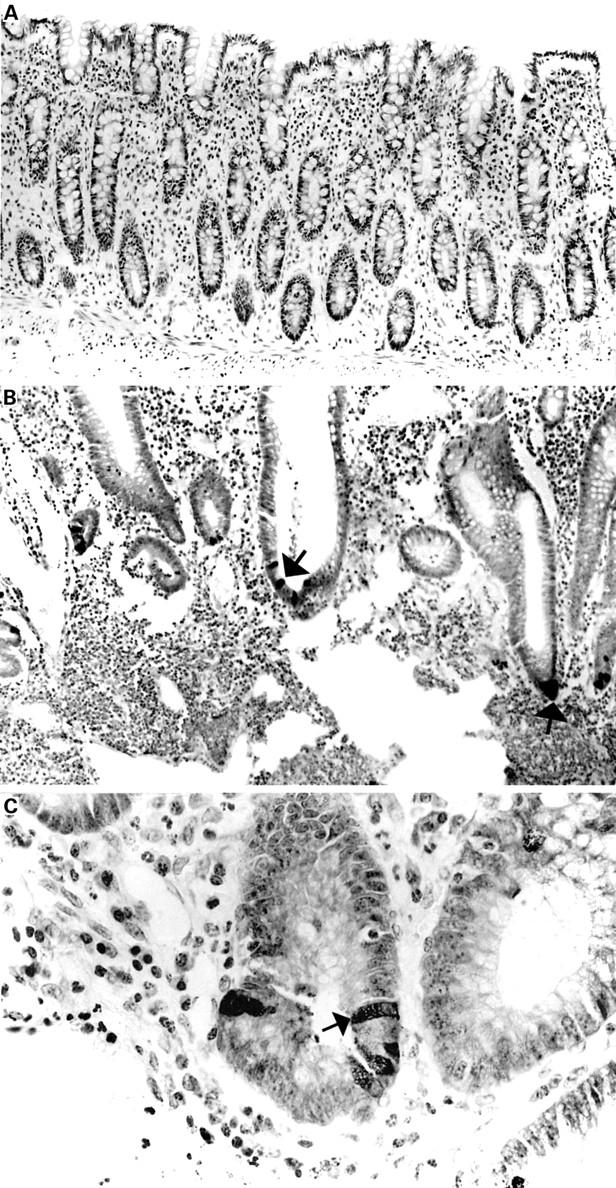
Sections of normal (A) and ulcerative colitis (B, C) colonic mucosal sections stained with anti-human defensin 5 (HD-5) antiserum. No HD-5 positive cells are present in (A). HD-5 immunoreactive epithelial cells (some of which are arrowed) are seen in the crypt regions of the sections with active ulcerative colitis (B, C).
Figure 6 .
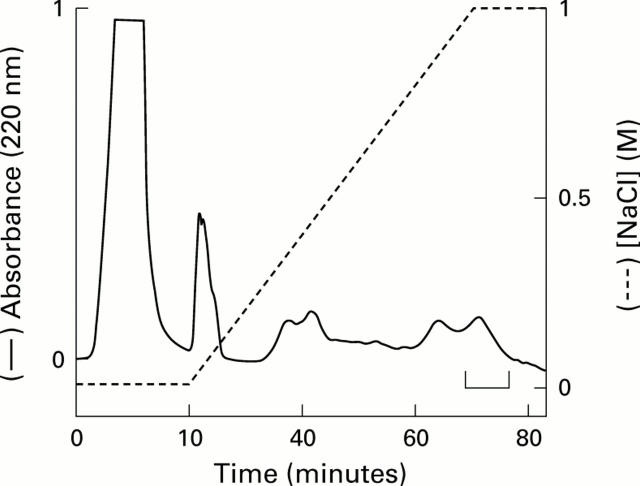
Cation exchange chromatography of ileal crypt cell extract. Acid extracts of terminal ileal crypt cells (obtained from five different resection specimens) were pooled and applied to a cation exchange column and eluted fractions containing human defensin 5 (HD-5) were identified by immunoblotting. Fractions eluting between 0.8 and 1 M NaCl demonstrated the strongest HD-5 immunoreactivity. Among these, fractions shown in the bracketed region were pooled for further purification by RP-HPLC.
Figure 7 .
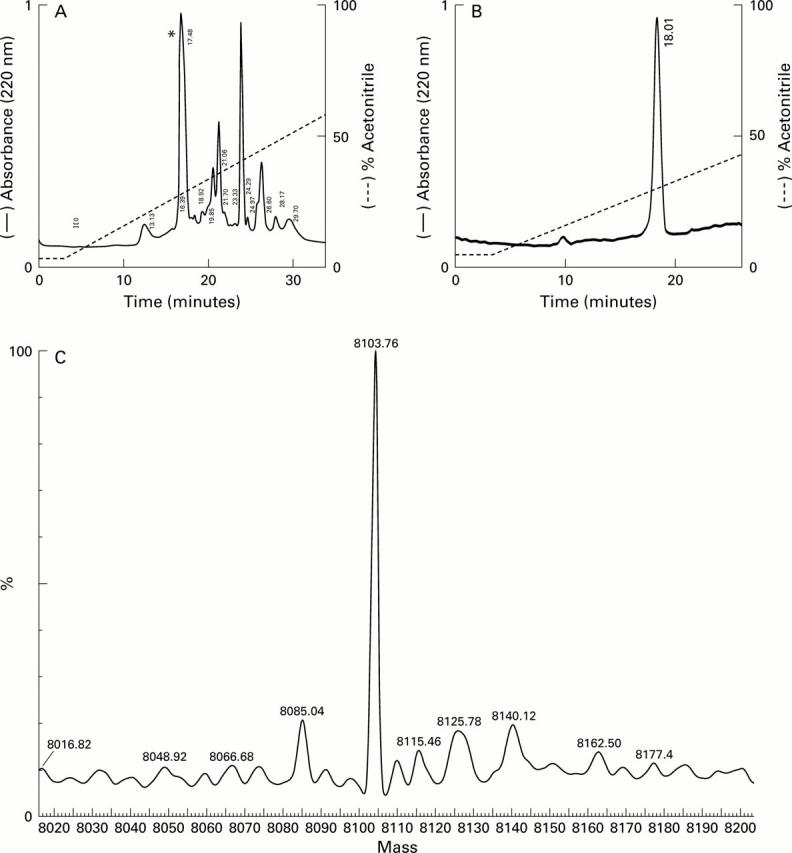
Purification and analysis of human defensin 5 (HD-5). (A) Pooled cation exchange fractions from the bracketed region in fig 6 were applied to a C-18 column. Individual eluted peaks were collected and screened for HD-5 immunoreactivity by immunoblotting. Only one fraction(*) was reactive. This fraction was collected and reapplied to the column and the elution profile (B) suggested the presence of a single entity. N-terminal amino acid sequencing of this fraction gave the sequence ESLQERADEA, corresponding to the predicted N-terminus of the precursor form of HD-5 (fig 1). (C) Mass spectral analysis of purified HD-5. The sample (same as in B) was analysed in a standard Qtof 1 instrument and performed in positive ion mode. The figure was obtained following transformation of raw data using the MaxEnt 1 algorithm and shows that the major component is a peptide with a predicted mass of 8103.76 Da. The mass of pro-HD-5 predicted from the amino acid sequence (20-94, see fig 1) is 8103.83 Da.
Figure 8 .
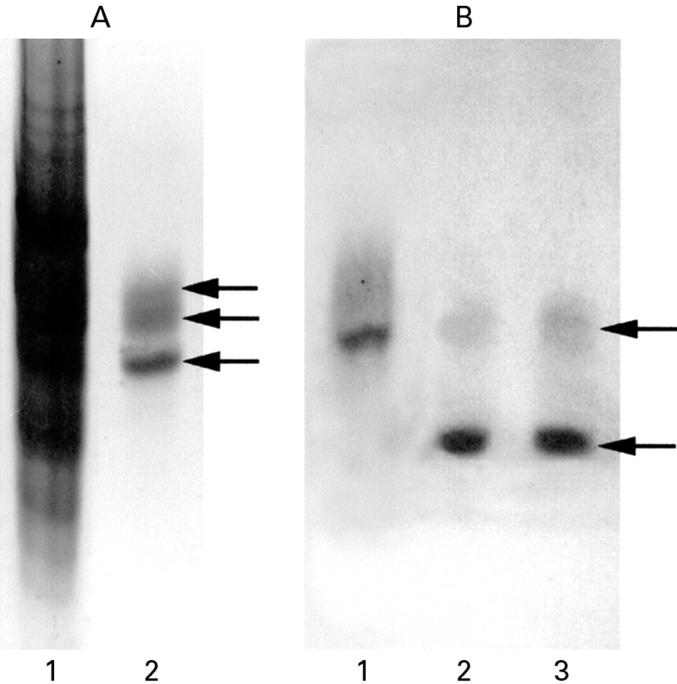
(A). AU-PAGE with Coomassie blue stain of crude acid extract of crypt epithelial cells (lane 1) and purified (by cation exchange chromatography and RP-HPLC; fig 7B) human defensin 5 (HD-5) (lane 2). HD-5 protein bands, as indicated by the arrows, were transferred to a PVDF membrane and sequenced directly. All three bands gave the identical N-terminal sequence of the precursor form of HD-5, ESLQERADEA. (B) AU-PAGE western blot analysis of HD-5 secreted by terminal ileal crypts. Supernatants obtained from isolated ileal crypts cultured for one hour in the presence of 10 µg/ml lipopolysaccharide (lane 2) or 10−4 M carbamylcholine chloride (lane 3) were subjected to AU-PAGE and transferred to a PVDF membrane and probed with anti-HD-5 antisera. Crude acid extract of crypt epithelial cells is included for comparison (lane 1). In addition to a band (upper arrow) corresponding to the precursor form of HD-5 (which is also seen in the crypt cell extract), an immunoreactive band of lower molecular weight (lower arrow) is present in the culture supernatants, corresponding to the truncated form of HD-5. Following RP-HPLC purification, N-terminal analysis of the truncated form of HD-5 revealed the sequence GEDNQDLAIS (amino acids 36-94).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Calvert R., Bordeleau G., Grondin G., Vezina A., Ferrari J. On the presence of intermediate cells in the small intestine. Anat Rec. 1988 Mar;220(3):291–295. doi: 10.1002/ar.1092200310. [DOI] [PubMed] [Google Scholar]
- Diamond G., Bevins C. L. beta-Defensins: endogenous antibiotics of the innate host defense response. Clin Immunol Immunopathol. 1998 Sep;88(3):221–225. doi: 10.1006/clin.1998.4587. [DOI] [PubMed] [Google Scholar]
- Eisenhauer P. B., Harwig S. S., Lehrer R. I. Cryptdins: antimicrobial defensins of the murine small intestine. Infect Immun. 1992 Sep;60(9):3556–3565. doi: 10.1128/iai.60.9.3556-3565.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elson C. O., Sartor R. B., Tennyson G. S., Riddell R. H. Experimental models of inflammatory bowel disease. Gastroenterology. 1995 Oct;109(4):1344–1367. doi: 10.1016/0016-5085(95)90599-5. [DOI] [PubMed] [Google Scholar]
- Fields P. I., Groisman E. A., Heffron F. A Salmonella locus that controls resistance to microbicidal proteins from phagocytic cells. Science. 1989 Feb 24;243(4894 Pt 1):1059–1062. doi: 10.1126/science.2646710. [DOI] [PubMed] [Google Scholar]
- Ganz T., Selsted M. E., Szklarek D., Harwig S. S., Daher K., Bainton D. F., Lehrer R. I. Defensins. Natural peptide antibiotics of human neutrophils. J Clin Invest. 1985 Oct;76(4):1427–1435. doi: 10.1172/JCI112120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geller S. A., Thung S. N. Morphologic unity of Paneth cells. Arch Pathol Lab Med. 1983 Sep;107(9):476–479. [PubMed] [Google Scholar]
- Harwig S. S., Tan L., Qu X. D., Cho Y., Eisenhauer P. B., Lehrer R. I. Bactericidal properties of murine intestinal phospholipase A2. J Clin Invest. 1995 Feb;95(2):603–610. doi: 10.1172/JCI117704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D. E., Bevins C. L. Defensin-6 mRNA in human Paneth cells: implications for antimicrobial peptides in host defense of the human bowel. FEBS Lett. 1993 Jan 4;315(2):187–192. doi: 10.1016/0014-5793(93)81160-2. [DOI] [PubMed] [Google Scholar]
- Jones D. E., Bevins C. L. Paneth cells of the human small intestine express an antimicrobial peptide gene. J Biol Chem. 1992 Nov 15;267(32):23216–23225. [PubMed] [Google Scholar]
- Lencer W. I., Cheung G., Strohmeier G. R., Currie M. G., Ouellette A. J., Selsted M. E., Madara J. L. Induction of epithelial chloride secretion by channel-forming cryptdins 2 and 3. Proc Natl Acad Sci U S A. 1997 Aug 5;94(16):8585–8589. doi: 10.1073/pnas.94.16.8585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahida Y. R., Galvin A. M., Gray T., Makh S., McAlindon M. E., Sewell H. F., Podolsky D. K. Migration of human intestinal lamina propria lymphocytes, macrophages and eosinophils following the loss of surface epithelial cells. Clin Exp Immunol. 1997 Aug;109(2):377–386. doi: 10.1046/j.1365-2249.1997.4481346.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahida Y. R., Rose F., Chan W. C. Antimicrobial peptides in the gastrointestinal tract. Gut. 1997 Feb;40(2):161–163. doi: 10.1136/gut.40.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason D. Y., Taylor C. R. The distribution of muramidase (lysozyme) in human tissues. J Clin Pathol. 1975 Feb;28(2):124–132. doi: 10.1136/jcp.28.2.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevalainen T. J., Grönroos J. M., Kallajoki M. Expression of group II phospholipase A2 in the human gastrointestinal tract. Lab Invest. 1995 Feb;72(2):201–208. [PubMed] [Google Scholar]
- Ouellette A. J., Hsieh M. M., Nosek M. T., Cano-Gauci D. F., Huttner K. M., Buick R. N., Selsted M. E. Mouse Paneth cell defensins: primary structures and antibacterial activities of numerous cryptdin isoforms. Infect Immun. 1994 Nov;62(11):5040–5047. doi: 10.1128/iai.62.11.5040-5047.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouellette A. J., Miller S. I., Henschen A. H., Selsted M. E. Purification and primary structure of murine cryptdin-1, a Paneth cell defensin. FEBS Lett. 1992 Jun 15;304(2-3):146–148. doi: 10.1016/0014-5793(92)80606-h. [DOI] [PubMed] [Google Scholar]
- Ouellette A. J. Paneth cells and innate immunity in the crypt microenvironment. Gastroenterology. 1997 Nov;113(5):1779–1784. doi: 10.1053/gast.1997.v113.pm9352884. [DOI] [PubMed] [Google Scholar]
- Podolsky D. K., Lynch-Devaney K., Stow J. L., Oates P., Murgue B., DeBeaumont M., Sands B. E., Mahida Y. R. Identification of human intestinal trefoil factor. Goblet cell-specific expression of a peptide targeted for apical secretion. J Biol Chem. 1993 Mar 25;268(9):6694–6702. [PubMed] [Google Scholar]
- Porter E. M., Liu L., Oren A., Anton P. A., Ganz T. Localization of human intestinal defensin 5 in Paneth cell granules. Infect Immun. 1997 Jun;65(6):2389–2395. doi: 10.1128/iai.65.6.2389-2395.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter E. M., Poles M. A., Lee J. S., Naitoh J., Bevins C. L., Ganz T. Isolation of human intestinal defensins from ileal neobladder urine. FEBS Lett. 1998 Sep 4;434(3):272–276. doi: 10.1016/s0014-5793(98)00994-6. [DOI] [PubMed] [Google Scholar]
- Porter E. M., van Dam E., Valore E. V., Ganz T. Broad-spectrum antimicrobial activity of human intestinal defensin 5. Infect Immun. 1997 Jun;65(6):2396–2401. doi: 10.1128/iai.65.6.2396-2401.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quayle A. J., Porter E. M., Nussbaum A. A., Wang Y. M., Brabec C., Yip K. P., Mok S. C. Gene expression, immunolocalization, and secretion of human defensin-5 in human female reproductive tract. Am J Pathol. 1998 May;152(5):1247–1258. [PMC free article] [PubMed] [Google Scholar]
- Rose F. R., Bailey K., Keyte J. W., Chan W. C., Greenwood D., Mahida Y. R. Potential role of epithelial cell-derived histone H1 proteins in innate antimicrobial defense in the human gastrointestinal tract. Infect Immun. 1998 Jul;66(7):3255–3263. doi: 10.1128/iai.66.7.3255-3263.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selsted M. E., Miller S. I., Henschen A. H., Ouellette A. J. Enteric defensins: antibiotic peptide components of intestinal host defense. J Cell Biol. 1992 Aug;118(4):929–936. doi: 10.1083/jcb.118.4.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troughton W. D., Trier J. S. Paneth and goblet cell renewal in mouse duodenal crypts. J Cell Biol. 1969 Apr;41(1):251–268. doi: 10.1083/jcb.41.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson C. L., Ouellette A. J., Satchell D. P., Ayabe T., López-Boado Y. S., Stratman J. L., Hultgren S. J., Matrisian L. M., Parks W. C. Regulation of intestinal alpha-defensin activation by the metalloproteinase matrilysin in innate host defense. Science. 1999 Oct 1;286(5437):113–117. doi: 10.1126/science.286.5437.113. [DOI] [PubMed] [Google Scholar]
- Zasloff M. Antibiotic peptides as mediators of innate immunity. Curr Opin Immunol. 1992 Feb;4(1):3–7. doi: 10.1016/0952-7915(92)90115-u. [DOI] [PubMed] [Google Scholar]