Abstract
BACKGROUND—Steroid dependent patients with Crohn's disease are at high risk of developing glucocorticosteroid induced side effects. AIMS—We evaluated the possibility of switching from systemic steroids to budesonide (Entocort) in prednisolone/prednisone dependent patients with inactive Crohn's disease affecting the ileum and/or ascending colon. PATIENTS—Steroid dependent patients with a Crohn's disease activity index ⩽200 were included. METHODS—In a double blind multicentre trial, 120 patients were randomly assigned to receive budesonide 6 mg once daily or placebo. Prednisolone was tapered to zero during the first 4-10 weeks and budesonide or placebo was given concomitantly and for a further 12 weeks. Relapse was defined as an index >200 and an increase of 60 points from baseline or withdrawal due to disease deterioration. RESULTS—After one and 13 weeks without prednisolone, relapse rates were 17% and 32%, respectively, in the budesonide group, and 41% and 65% in the placebo group (95% confidence intervals for the difference in percentages −41%, −8% and −51%, −16%; p=0.004 and p<0.001, respectively). The number of glucocorticosteroid side effects was reduced by 50% by switching from prednisolone and was similar in the budesonide and placebo groups. Basal plasma cortisol increased in both groups. CONCLUSIONS—The majority of patients with steroid dependent ileocaecal Crohn's disease may be switched to budesonide controlled ileal release capsules 6 mg without relapse, resulting in a sharp decrease in glucocorticosteroid side effects similar to placebo, and with an increase in plasma cortisol levels. Keywords: budesonide; Crohn's disease; steroid dependent; prednisolone
Full Text
The Full Text of this article is available as a PDF (148.7 KB).
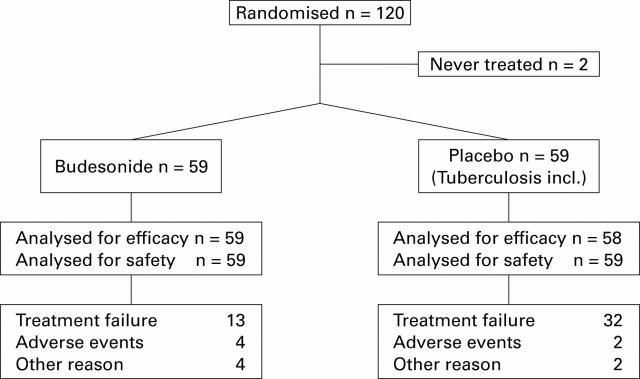
Figure 1 .
Flow chart of patients and reasons for discontinuation.
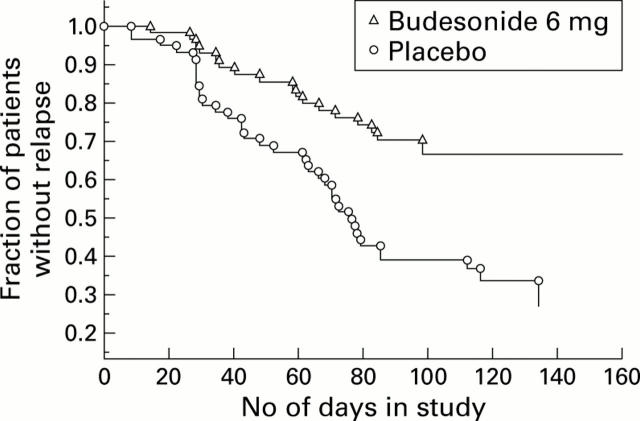
Figure 2 .
Time to relapse in the budesonide and placebo groups.
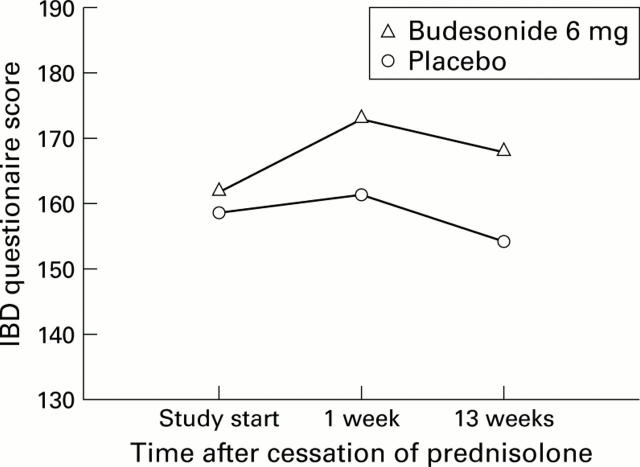
Figure 3 .
Scores from the inflammatory bowel disease (IBD) questionnaire for the budesonide and placebo groups.
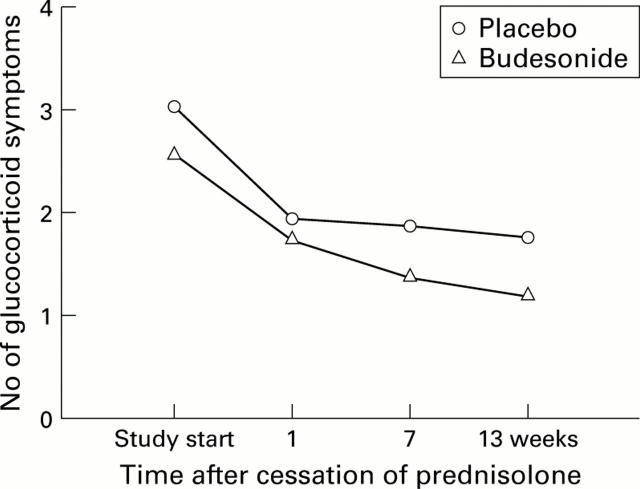
Figure 4 .
Number of glucocorticosteroid related symptoms in the budesonide and placebo groups.
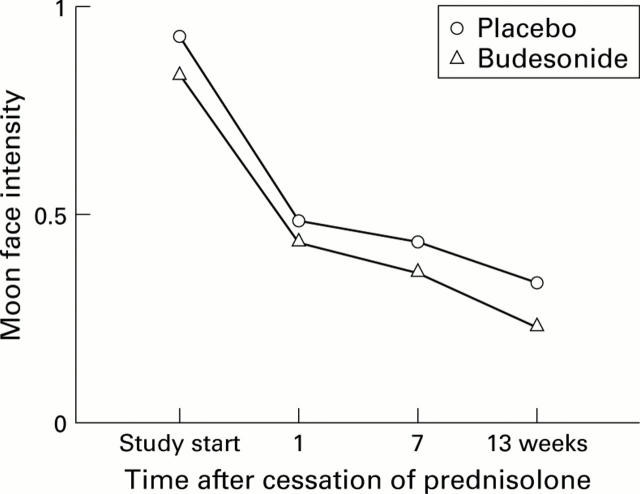
Figure 5 .
Average intensity of moon face at each visit in the budesonide and placebo groups.
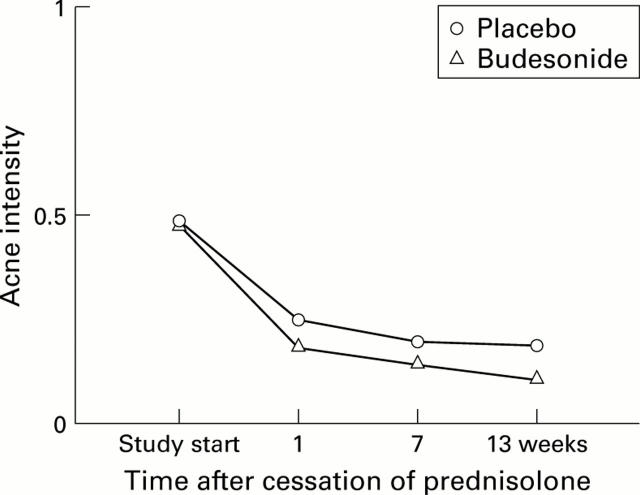
Figure 6 .
Average intensity of acne at each visit in the budesonide and placebo groups.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Best W. R., Becktel J. M., Singleton J. W., Kern F., Jr Development of a Crohn's disease activity index. National Cooperative Crohn's Disease Study. Gastroenterology. 1976 Mar;70(3):439–444. [PubMed] [Google Scholar]
- Campieri M., Ferguson A., Doe W., Persson T., Nilsson L. G. Oral budesonide is as effective as oral prednisolone in active Crohn's disease. The Global Budesonide Study Group. Gut. 1997 Aug;41(2):209–214. doi: 10.1136/gut.41.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feagan B. G., Rochon J., Fedorak R. N., Irvine E. J., Wild G., Sutherland L., Steinhart A. H., Greenberg G. R., Gillies R., Hopkins M. Methotrexate for the treatment of Crohn's disease. The North American Crohn's Study Group Investigators. N Engl J Med. 1995 Feb 2;332(5):292–297. doi: 10.1056/NEJM199502023320503. [DOI] [PubMed] [Google Scholar]
- Greenberg G. R., Feagan B. G., Martin F., Sutherland L. R., Thomson A. B., Williams C. N., Nilsson L. G., Persson T. Oral budesonide as maintenance treatment for Crohn's disease: a placebo-controlled, dose-ranging study. Canadian Inflammatory Bowel Disease Study Group. Gastroenterology. 1996 Jan;110(1):45–51. doi: 10.1053/gast.1996.v110.pm8536887. [DOI] [PubMed] [Google Scholar]
- Greenberg G. R., Feagan B. G., Martin F., Sutherland L. R., Thomson A. B., Williams C. N., Nilsson L. G., Persson T. Oral budesonide for active Crohn's disease. Canadian Inflammatory Bowel Disease Study Group. N Engl J Med. 1994 Sep 29;331(13):836–841. doi: 10.1056/NEJM199409293311303. [DOI] [PubMed] [Google Scholar]
- Guyatt G., Mitchell A., Irvine E. J., Singer J., Williams N., Goodacre R., Tompkins C. A new measure of health status for clinical trials in inflammatory bowel disease. Gastroenterology. 1989 Mar;96(3):804–810. [PubMed] [Google Scholar]
- Löfberg R., Rutgeerts P., Malchow H., Lamers C., Danielsson A., Olaison G., Jewell D., Ostergaard Thomsen O., Lorenz-Meyer H., Goebell H. Budesonide prolongs time to relapse in ileal and ileocaecal Crohn's disease. A placebo controlled one year study. Gut. 1996 Jul;39(1):82–86. doi: 10.1136/gut.39.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers S., Sachar D. B. Medical management of Crohn's disease. Hepatogastroenterology. 1990 Feb;37(1):42–55. [PubMed] [Google Scholar]
- Munkholm P., Langholz E., Davidsen M., Binder V. Frequency of glucocorticoid resistance and dependency in Crohn's disease. Gut. 1994 Mar;35(3):360–362. doi: 10.1136/gut.35.3.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson D. C., May G. R., Fick G. H., Sutherland L. R. Azathioprine and 6-mercaptopurine in Crohn disease. A meta-analysis. Ann Intern Med. 1995 Jul 15;123(2):132–142. doi: 10.7326/0003-4819-123-2-199507150-00009. [DOI] [PubMed] [Google Scholar]
- Rutgeerts P., Löfberg R., Malchow H., Lamers C., Olaison G., Jewell D., Danielsson A., Goebell H., Thomsen O. O., Lorenz-Meyer H. A comparison of budesonide with prednisolone for active Crohn's disease. N Engl J Med. 1994 Sep 29;331(13):842–845. doi: 10.1056/NEJM199409293311304. [DOI] [PubMed] [Google Scholar]
- Rutgeerts P. Medical therapy of inflammatory bowel disease. Digestion. 1998 Aug;59(5):453–469. doi: 10.1159/000007523. [DOI] [PubMed] [Google Scholar]
- Shapiro G., Mendelson L., Kraemer M. J., Cruz-Rivera M., Walton-Bowen K., Smith J. A. Efficacy and safety of budesonide inhalation suspension (Pulmicort Respules) in young children with inhaled steroid-dependent, persistent asthma. J Allergy Clin Immunol. 1998 Nov;102(5):789–796. doi: 10.1016/s0091-6749(98)70019-3. [DOI] [PubMed] [Google Scholar]
- Summers R. W., Switz D. M., Sessions J. T., Jr, Becktel J. M., Best W. R., Kern F., Jr, Singleton J. W. National Cooperative Crohn's Disease Study: results of drug treatment. Gastroenterology. 1979 Oct;77(4 Pt 2):847–869. [PubMed] [Google Scholar]
- Ware J. E., Jr, Sherbourne C. D. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992 Jun;30(6):473–483. [PubMed] [Google Scholar]