Abstract
BACKGROUND—The normal intestinal microflora plays an important role in host metabolism and provides a natural defence mechanism against invading pathogens. Although the microbiota in adults has been extensively studied, little is known of the changes that occur in the microflora with aging. These may have important consequences in elderly people, many of whom are receiving antibiotic therapy and who are most susceptible to intestinal dysbiosis. AIMS—To characterise the major groups of faecal bacteria in subjects of different ages using a combination of cultural, molecular, and chemotaxonomic approaches. METHODS—Comparative microbiological studies were made on four different subject groups: children (16 months to seven years, n=10), adults (21-34 years, n=7), healthy elderly subjects (67-88 years, n=5), and geriatric patients (68-73 years, n=4) diagnosed with Clostridium difficile diarrhoea. Selected faecal bacteria were investigated using viable counting procedures, 16S ribosomal RNA (rRNA) abundance measurements, and the occurrence of specific signature fatty acids in whole community fatty acid methyl ester profiles. RESULTS—The principal microbiological difference between adults and children was the occurrence of higher numbers of enterobacteria in the latter group, as determined by viable counts (p<0.05) and 16S rRNA (p<0.01) measurements. Moreover, a greater proportion of children's faecal rRNA was hybridised by the three probes (bifidobacteria, enterobacteria, bacteroides-porphyromonas-prevotella) used in the study, indicating a less developed gut microbiota. Species diversity was also markedly lower in the Clostridium difficile associated diarrhoea group, which was characterised by high numbers of facultative anaerobes and low levels of bifidobacteria and bacteroides. Although it was a considerably less sensitive diagnostic tool, cellular fatty acid analysis correlated with viable bacterial counts and 16S rRNA measurements in a number of bacteria, including bacteroides. CONCLUSIONS—Polyphasic analysis of faecal bacteria showed that significant structural changes occur in the microbiota with aging, and this was especially evident with respect to putatively protective bifidobacteria. Reductions in these organisms in the large bowel may be related to increased disease risk in elderly people. Keywords: intestinal bacteria; aging; cellular fatty acids; ribosomal RNA; Clostridium difficile infection
Full Text
The Full Text of this article is available as a PDF (175.8 KB).
Figure 1 .
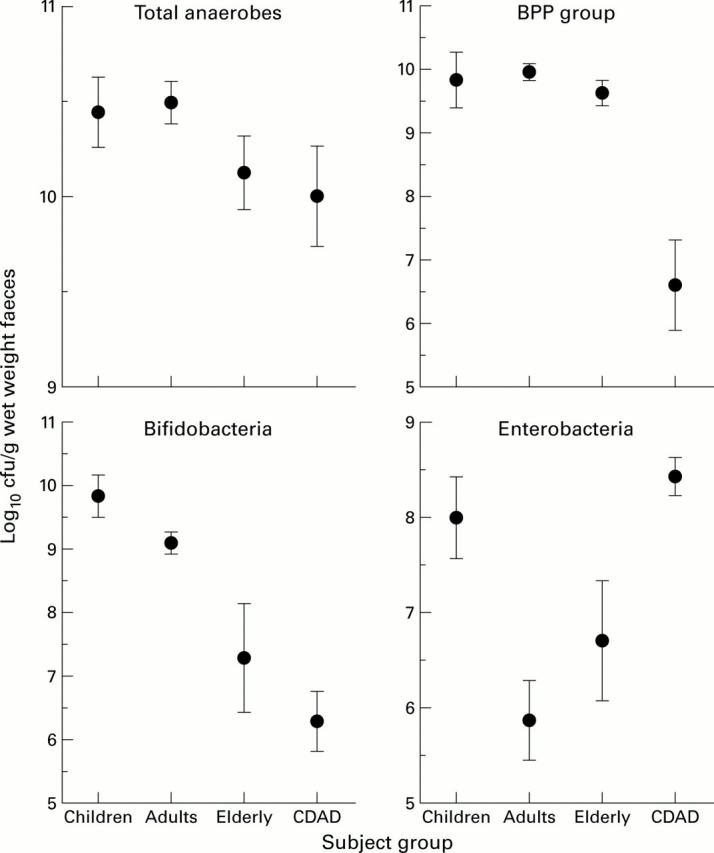
Viable counts of bacteroides, bifidobacteria, enterobacteria, and total anaerobes in faeces of different age groups. Results are mean (SEM) in children (n=5), adults (n=7), elderly subjects (n=4), and in patients with Clostridium difficile associated diarrhoea (CDAD, n=4). BPP, Bacteroides-Porphyromonas-Prevotella.
Figure 2 .
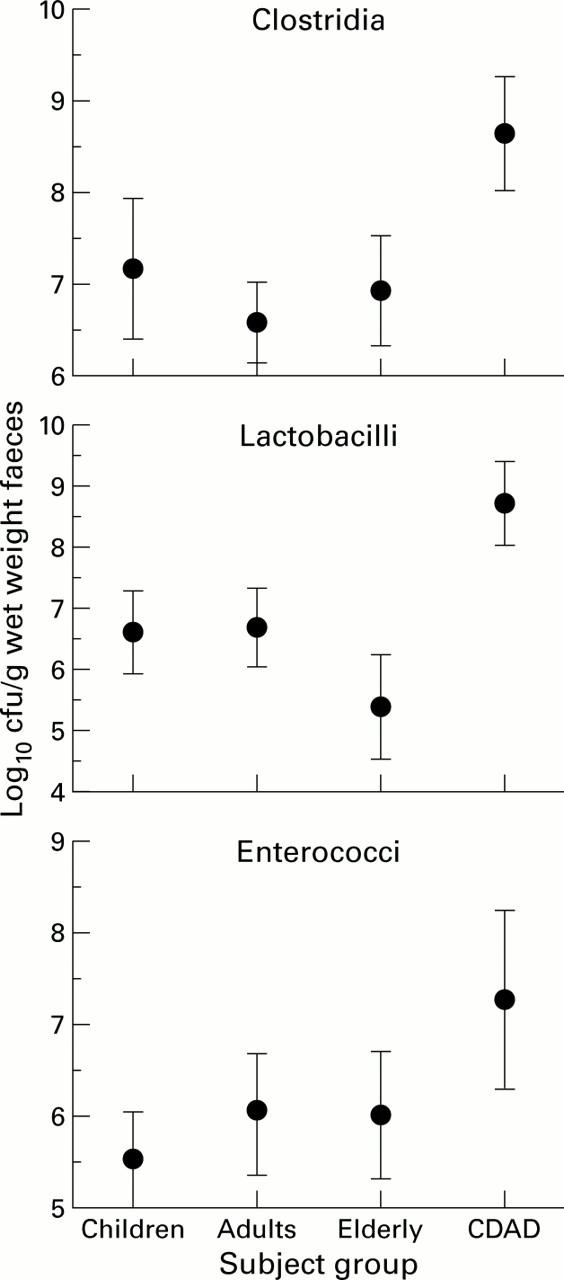
Viable counts of clostridia, lactobacilli, and enterococci in faeces obtained from children (n=5), adults (n=7), elderly subjects (n=4), and patients with Clostridium difficile associated diarrhoeal (CDAD, n=4).
Figure 3 .
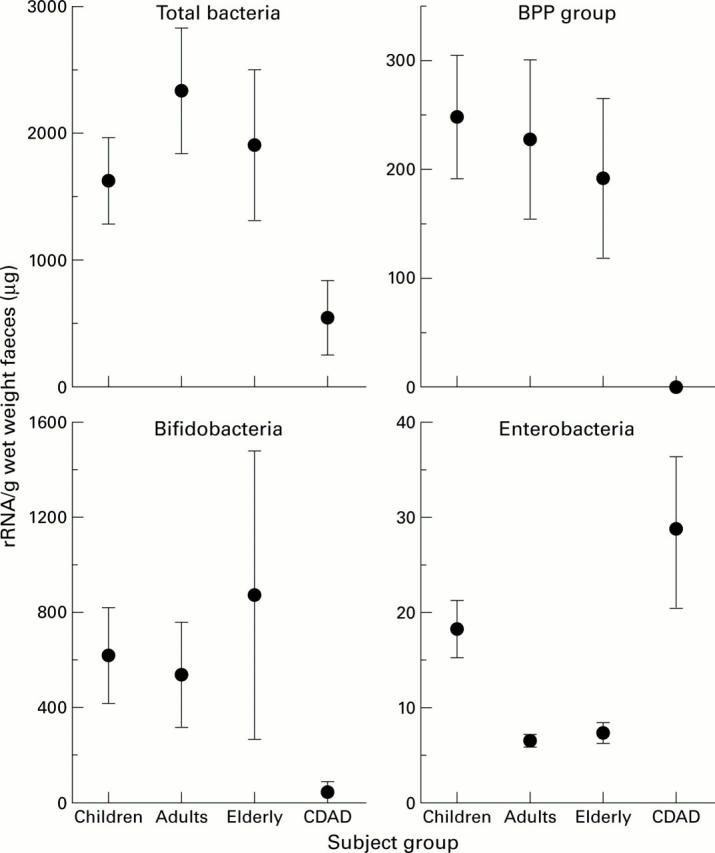
16S rRNA concentrations in faeces. Results are mean (SEM) in children (n=10), adults (n=7), elderly subjects (n=5), and in patients with C difficile associated diarrhoea (CDAD, n=4). BPP, Bacteroides-Porphyromonas-Prevotella.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amann R. I., Binder B. J., Olson R. J., Chisholm S. W., Devereux R., Stahl D. A. Combination of 16S rRNA-targeted oligonucleotide probes with flow cytometry for analyzing mixed microbial populations. Appl Environ Microbiol. 1990 Jun;56(6):1919–1925. doi: 10.1128/aem.56.6.1919-1925.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benno Y., Nakao H., Uchida K., Mitsuoka T. Impact of the advances in age on the gastrointestinal microflora of beagle dogs. J Vet Med Sci. 1992 Aug;54(4):703–706. doi: 10.1292/jvms.54.703. [DOI] [PubMed] [Google Scholar]
- Campbell J. M., Fahey G. C., Jr, Lichtensteiger C. A., Demichele S. J., Garleb K. A. An enteral formula containing fish oil, indigestible oligosaccharides, gum arabic and antioxidants affects plasma and colonic phospholipid fatty acid and prostaglandin profiles in pigs. J Nutr. 1997 Jan;127(1):137–145. doi: 10.1093/jn/127.1.137. [DOI] [PubMed] [Google Scholar]
- Doré J., Sghir A., Hannequart-Gramet G., Corthier G., Pochart P. Design and evaluation of a 16S rRNA-targeted oligonucleotide probe for specific detection and quantitation of human faecal Bacteroides populations. Syst Appl Microbiol. 1998 Mar;21(1):65–71. doi: 10.1016/S0723-2020(98)80009-X. [DOI] [PubMed] [Google Scholar]
- Eerola E., Möttönen T., Hannonen P., Luukkainen R., Kantola I., Vuori K., Tuominen J., Toivanen P. Intestinal flora in early rheumatoid arthritis. Br J Rheumatol. 1994 Nov;33(11):1030–1038. doi: 10.1093/rheumatology/33.11.1030. [DOI] [PubMed] [Google Scholar]
- Ellis-Pegler R. B., Crabtree C., Lambert H. P. The faecal flora of children in the United Kingdom. J Hyg (Lond) 1975 Aug;75(1):135–142. doi: 10.1017/s002217240004715x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks A. H., Harmsen H. J., Raangs G. C., Jansen G. J., Schut F., Welling G. W. Variations of bacterial populations in human feces measured by fluorescent in situ hybridization with group-specific 16S rRNA-targeted oligonucleotide probes. Appl Environ Microbiol. 1998 Sep;64(9):3336–3345. doi: 10.1128/aem.64.9.3336-3345.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson G. R., Beatty E. R., Wang X., Cummings J. H. Selective stimulation of bifidobacteria in the human colon by oligofructose and inulin. Gastroenterology. 1995 Apr;108(4):975–982. doi: 10.1016/0016-5085(95)90192-2. [DOI] [PubMed] [Google Scholar]
- Gibson G. R., Roberfroid M. B. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr. 1995 Jun;125(6):1401–1412. doi: 10.1093/jn/125.6.1401. [DOI] [PubMed] [Google Scholar]
- Gibson G. R., Wang X. Regulatory effects of bifidobacteria on the growth of other colonic bacteria. J Appl Bacteriol. 1994 Oct;77(4):412–420. doi: 10.1111/j.1365-2672.1994.tb03443.x. [DOI] [PubMed] [Google Scholar]
- Gorbach S. L., Nahas L., Lerner P. I., Weinstein L. Studies of intestinal microflora. I. Effects of diet, age, and periodic sampling on numbers of fecal microorganisms in man. Gastroenterology. 1967 Dec;53(6):845–855. [PubMed] [Google Scholar]
- Haack S. K., Garchow H., Odelson D. A., Forney L. J., Klug M. J. Accuracy, reproducibility, and interpretation of Fatty Acid methyl ester profiles of model bacterial communities. Appl Environ Microbiol. 1994 Jul;60(7):2483–2493. doi: 10.1128/aem.60.7.2483-2493.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langendijk P. S., Schut F., Jansen G. J., Raangs G. C., Kamphuis G. R., Wilkinson M. H., Welling G. W. Quantitative fluorescence in situ hybridization of Bifidobacterium spp. with genus-specific 16S rRNA-targeted probes and its application in fecal samples. Appl Environ Microbiol. 1995 Aug;61(8):3069–3075. doi: 10.1128/aem.61.8.3069-3075.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljungberg B., Nilsson-Ehle I., Edlund C., Nord C. E. Influence of ciprofloxacin on the colonic microflora in young and elderly volunteers: no impact of the altered drug absorption. Scand J Infect Dis. 1990;22(2):205–208. doi: 10.3109/00365549009037903. [DOI] [PubMed] [Google Scholar]
- Lovat L. B. Age related changes in gut physiology and nutritional status. Gut. 1996 Mar;38(3):306–309. doi: 10.1136/gut.38.3.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macfarlane G. T., Cummings J. H., Allison C. Protein degradation by human intestinal bacteria. J Gen Microbiol. 1986 Jun;132(6):1647–1656. doi: 10.1099/00221287-132-6-1647. [DOI] [PubMed] [Google Scholar]
- Macfarlane G. T., Cummings J. H. Probiotics and prebiotics: can regulating the activities of intestinal bacteria benefit health? BMJ. 1999 Apr 10;318(7189):999–1003. doi: 10.1136/bmj.318.7189.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton D. F., Cummings J. H., Macfarlane S., Macfarlane G. T. Growth of a human intestinal Desulfovibrio desulfuricans in continuous cultures containing defined populations of saccharolytic and amino acid fermenting bacteria. J Appl Microbiol. 1998 Aug;85(2):372–380. doi: 10.1046/j.1365-2672.1998.00522.x. [DOI] [PubMed] [Google Scholar]
- Peltonen R., Ling W. H., Hänninen O., Eerola E. An uncooked vegan diet shifts the profile of human fecal microflora: computerized analysis of direct stool sample gas-liquid chromatography profiles of bacterial cellular fatty acids. Appl Environ Microbiol. 1992 Nov;58(11):3660–3666. doi: 10.1128/aem.58.11.3660-3666.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltonen R., Nenonen M., Helve T., Hänninen O., Toivanen P., Eerola E. Faecal microbial flora and disease activity in rheumatoid arthritis during a vegan diet. Br J Rheumatol. 1997 Jan;36(1):64–68. doi: 10.1093/rheumatology/36.1.64. [DOI] [PubMed] [Google Scholar]
- Percival R. S., Marsh P. D., Challacombe S. J. Serum antibodies to commensal oral and gut bacteria vary with age. FEMS Immunol Med Microbiol. 1996 Aug;15(1):35–42. doi: 10.1111/j.1574-695X.1996.tb00356.x. [DOI] [PubMed] [Google Scholar]
- Pollard A. J., Galassini R., Rouppe van der Voort E. M., Hibberd M., Booy R., Langford P., Nadel S., Ison C., Kroll J. S., Poolman J. Cellular immune responses to Neisseria meningitidis in children. Infect Immun. 1999 May;67(5):2452–2463. doi: 10.1128/iai.67.5.2452-2463.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard A. J., Galassini R., van der Voort E. M., Booy R., Langford P., Nadel S., Ison C., Kroll J. S., Poolman J., Levin M. Humoral immune responses to Neisseria meningitidis in children. Infect Immun. 1999 May;67(5):2441–2451. doi: 10.1128/iai.67.5.2441-2451.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raskin L., Poulsen L. K., Noguera D. R., Rittmann B. E., Stahl D. A. Quantification of methanogenic groups in anaerobic biological reactors by oligonucleotide probe hybridization. Appl Environ Microbiol. 1994 Apr;60(4):1241–1248. doi: 10.1128/aem.60.4.1241-1248.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultsz C., Van Den Berg F. M., Ten Kate F. W., Tytgat G. N., Dankert J. The intestinal mucus layer from patients with inflammatory bowel disease harbors high numbers of bacteria compared with controls. Gastroenterology. 1999 Nov;117(5):1089–1097. doi: 10.1016/s0016-5085(99)70393-8. [DOI] [PubMed] [Google Scholar]
- Sghir A., Gramet G., Suau A., Rochet V., Pochart P., Dore J. Quantification of bacterial groups within human fecal flora by oligonucleotide probe hybridization. Appl Environ Microbiol. 2000 May;66(5):2263–2266. doi: 10.1128/aem.66.5.2263-2266.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl D. A., Flesher B., Mansfield H. R., Montgomery L. Use of phylogenetically based hybridization probes for studies of ruminal microbial ecology. Appl Environ Microbiol. 1988 May;54(5):1079–1084. doi: 10.1128/aem.54.5.1079-1084.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark P. L., Lee A. The microbial ecology of the large bowel of breast-fed and formula-fed infants during the first year of life. J Med Microbiol. 1982 May;15(2):189–203. doi: 10.1099/00222615-15-2-189. [DOI] [PubMed] [Google Scholar]
- Suau A., Bonnet R., Sutren M., Godon J. J., Gibson G. R., Collins M. D., Doré J. Direct analysis of genes encoding 16S rRNA from complex communities reveals many novel molecular species within the human gut. Appl Environ Microbiol. 1999 Nov;65(11):4799–4807. doi: 10.1128/aem.65.11.4799-4807.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tojo M., Oikawa T., Morikawa Y., Yamashita N., Iwata S., Satoh Y., Hanada J., Tanaka R. The effects of Bifidobacterium breve administration on campylobacter enteritis. Acta Paediatr Jpn. 1987 Feb;29(1):160–167. doi: 10.1111/j.1442-200x.1987.tb00024.x. [DOI] [PubMed] [Google Scholar]
- Waterlow J. C. Protein-energy malnutrition: the nature and extent of the problem. Clin Nutr. 1997 Mar;16 (Suppl 1):3–9. doi: 10.1016/s0261-5614(97)80043-x. [DOI] [PubMed] [Google Scholar]
- Welling G. W., Elfferich P., Raangs G. C., Wildeboer-Veloo A. C., Jansen G. J., Degener J. E. 16S ribosomal RNA-targeted oligonucleotide probes for monitoring of intestinal tract bacteria. Scand J Gastroenterol Suppl. 1997;222:17–19. doi: 10.1080/00365521.1997.11720711. [DOI] [PubMed] [Google Scholar]
- Yamazaki S., Machii K., Tsuyuki S., Momose H., Kawashima T., Ueda K. Immunological responses to monoassociated Bifidobacterium longum and their relation to prevention of bacterial invasion. Immunology. 1985 Sep;56(1):43–50. [PMC free article] [PubMed] [Google Scholar]


