Abstract
BACKGROUND AND AIM—Indirect evidence suggests that CD4+ T cells have a pathogenic while γδ T cells have a protective role in the initiation and perpetuation of inflammatory bowel disease. To define the role of T cell subsets in a rat colitis model (2,4,6-trinitrobenzene sulphonic acid (TNBS)) we analysed colitis severity after effective depletion of T helper cells, αβ T cells, or γδ T cells. METHODS—T helper cells, αβ T cells, or γδ T cells were depleted using previously described monoclonal antibodies directed at the CD4 molecule (OX38), the CD2 molecule (OX34, both depleting CD4+ T cells), the αβ T cell receptor (R73), and the γδ T cell receptor (V65). Depletion was verified by flow cytometry and/or immunohistology. Colitis was induced using intracolonic application of TNBS. RESULTS—Surprisingly, depletion of T helper cells or αβ T cells had no influence on survival, macroscopic or microscopic scores, or myeloperoxidase activity following colitis induction. In contrast, depletion of γδ T cells resulted in significantly increased mortality (V65: 73%, n=15) compared with controls (30%, n=13; p<0.03). In addition, colitis was histologically more severe in the γδ T cell depleted group compared with controls (p<0.05). CONCLUSIONS—T helper cells or αβ T cells did not influence the initiation or perpetuation of rat TNBS colitis. In contrast, γδ T cells had a protective role in rat TNBS colitis as depletion caused increased mortality. Keywords: αβ T cells; γδ T cells; experimental colitis; inflammatory bowel disease; rat
Full Text
The Full Text of this article is available as a PDF (169.9 KB).
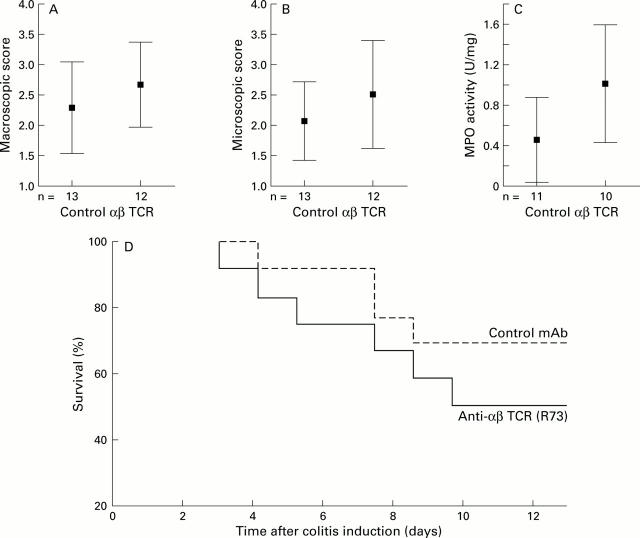
Figure 1 .
No significant effect of αβ T cell depletion on colitis severity. Mean (95% confidence interval) values for (A) macroscopic score, (B) microscopic score, (C) myeloperoxidase (MPO) activity, and (D) survival (Kaplan-Meier analysis) comparing isotype matched control monoclonal antibody (mAb) (TS2/9, n=13) and the anti-αβ T cell receptor (TCR) mAb (R73, n=12). A dose of 200 µg followed by 100 µg of each mAb was started two days prior to colitis induction and continued on days −1, 0, 2, 4, 6, 8, and 10 after colitis induction.
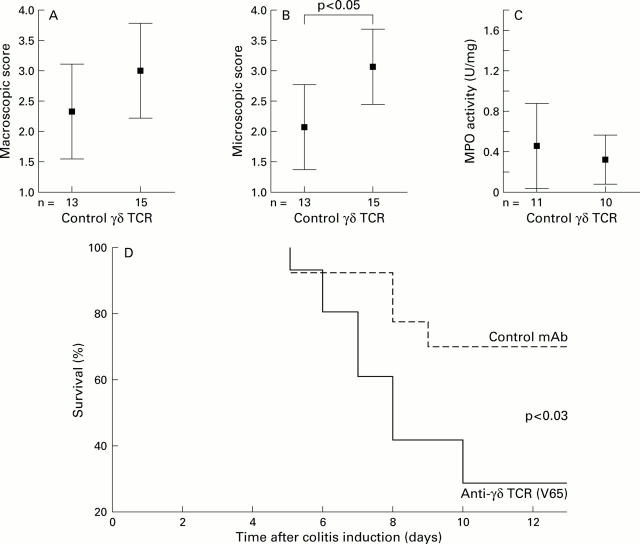
Figure 2 .
Increased mortality of γδ T cell depleted rats with TNBS colitis. Mean (95% confidence interval) values for (A) macroscopic score, (B) microscopic score, (C) myeloperoxidase (MPO) activity, and (D) survival (Kaplan-Meier analysis) comparing isotype matched control monoclonal antibody (mAb) (TS2/9, n=13) and the anti-γδ T cell receptor (TCR) mAb (V65, n=15).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison J. P., Havran W. L. The immunobiology of T cells with invariant gamma delta antigen receptors. Annu Rev Immunol. 1991;9:679–705. doi: 10.1146/annurev.iy.09.040191.003335. [DOI] [PubMed] [Google Scholar]
- Blumberg R. S., Lencer W. I., Zhu X., Kim H. S., Claypool S., Balk S. P., Saubermann L. J., Colgan S. P. Antigen presentation by intestinal epithelial cells. Immunol Lett. 1999 Jun 15;69(1):7–11. doi: 10.1016/s0165-2478(99)00093-0. [DOI] [PubMed] [Google Scholar]
- Bucht A., Söderström K., Esin S., Grunewald J., Hagelberg S., Magnusson I., Wigzell H., Grönberg A., Kiessling R. Analysis of gamma delta V region usage in normal and diseased human intestinal biopsies and peripheral blood by polymerase chain reaction (PCR) and flow cytometry. Clin Exp Immunol. 1995 Jan;99(1):57–64. doi: 10.1111/j.1365-2249.1995.tb03472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contractor N. V., Bassiri H., Reya T., Park A. Y., Baumgart D. C., Wasik M. A., Emerson S. G., Carding S. R. Lymphoid hyperplasia, autoimmunity, and compromised intestinal intraepithelial lymphocyte development in colitis-free gnotobiotic IL-2-deficient mice. J Immunol. 1998 Jan 1;160(1):385–394. [PubMed] [Google Scholar]
- Dengler T. J., Hoffmann J. C., Knolle P., Albert-Wolf M., Roux M., Wallich R., Meuer S. C. Structural and functional epitopes of the human adhesion receptor CD58 (LFA-3). Eur J Immunol. 1992 Nov;22(11):2809–2817. doi: 10.1002/eji.1830221109. [DOI] [PubMed] [Google Scholar]
- Dohi T., Fujihashi K., Rennert P. D., Iwatani K., Kiyono H., McGhee J. R. Hapten-induced colitis is associated with colonic patch hypertrophy and T helper cell 2-type responses. J Exp Med. 1999 Apr 19;189(8):1169–1180. doi: 10.1084/jem.189.8.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elson C. O., Sartor R. B., Tennyson G. S., Riddell R. H. Experimental models of inflammatory bowel disease. Gastroenterology. 1995 Oct;109(4):1344–1367. doi: 10.1016/0016-5085(95)90599-5. [DOI] [PubMed] [Google Scholar]
- Fujihashi K., Taguchi T., Aicher W. K., McGhee J. R., Bluestone J. A., Eldridge J. H., Kiyono H. Immunoregulatory functions for murine intraepithelial lymphocytes: gamma/delta T cell receptor-positive (TCR+) T cells abrogate oral tolerance, while alpha/beta TCR+ T cells provide B cell help. J Exp Med. 1992 Mar 1;175(3):695–707. doi: 10.1084/jem.175.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima K., Masuda T., Ohtani H., Sasaki I., Funayama Y., Matsuno S., Nagura H. Immunohistochemical characterization, distribution, and ultrastructure of lymphocytes bearing T-cell receptor gamma/delta in inflammatory bowel disease. Gastroenterology. 1991 Sep;101(3):670–678. doi: 10.1016/0016-5085(91)90524-o. [DOI] [PubMed] [Google Scholar]
- Goodman T., Lefrançois L. Expression of the gamma-delta T-cell receptor on intestinal CD8+ intraepithelial lymphocytes. Nature. 1988 Jun 30;333(6176):855–858. doi: 10.1038/333855a0. [DOI] [PubMed] [Google Scholar]
- Grisham M. B., Benoit J. N., Granger D. N. Assessment of leukocyte involvement during ischemia and reperfusion of intestine. Methods Enzymol. 1990;186:729–742. doi: 10.1016/0076-6879(90)86172-r. [DOI] [PubMed] [Google Scholar]
- Harrison L. C., Dempsey-Collier M., Kramer D. R., Takahashi K. Aerosol insulin induces regulatory CD8 gamma delta T cells that prevent murine insulin-dependent diabetes. J Exp Med. 1996 Dec 1;184(6):2167–2174. doi: 10.1084/jem.184.6.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helgeland L., Vaage J. T., Rolstad B., Halstensen T. S., Midtvedt T., Brandtzaeg P. Regional phenotypic specialization of intraepithelial lymphocytes in the rat intestine does not depend on microbial colonization. Scand J Immunol. 1997 Oct;46(4):349–357. doi: 10.1046/j.1365-3083.1997.d01-133.x. [DOI] [PubMed] [Google Scholar]
- Higgins L. M., McDonald S. A., Whittle N., Crockett N., Shields J. G., MacDonald T. T. Regulation of T cell activation in vitro and in vivo by targeting the OX40-OX40 ligand interaction: amelioration of ongoing inflammatory bowel disease with an OX40-IgG fusion protein, but not with an OX40 ligand-IgG fusion protein. J Immunol. 1999 Jan 1;162(1):486–493. [PubMed] [Google Scholar]
- Hoffmann J. C., Herklotz C., Zeidler H., Bayer B., Rosenthal H., Westermann J. Initiation and perpetuation of rat adjuvant arthritis is inhibited by the anti-CD2 monoclonal antibody (mAb) OX34. Ann Rheum Dis. 1997 Dec;56(12):716–722. doi: 10.1136/ard.56.12.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann J. C., Herklotz C., Zeidler H., Bayer B., Westermann J. Anti-CD2 (OX34) MoAb treatment of adjuvant arthritic rats: attenuation of established arthritis, selective depletion of CD4+ T cells, and CD2 down-modulation. Clin Exp Immunol. 1997 Oct;110(1):63–71. doi: 10.1046/j.1365-2249.1997.4881385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hünig T., Wallny H. J., Hartley J. K., Lawetzky A., Tiefenthaler G. A monoclonal antibody to a constant determinant of the rat T cell antigen receptor that induces T cell activation. Differential reactivity with subsets of immature and mature T lymphocytes. J Exp Med. 1989 Jan 1;169(1):73–86. doi: 10.1084/jem.169.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann S. H. gamma/delta and other unconventional T lymphocytes: what do they see and what do they do? Proc Natl Acad Sci U S A. 1996 Mar 19;93(6):2272–2279. doi: 10.1073/pnas.93.6.2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke Y., Pearce K., Lake J. P., Ziegler H. K., Kapp J. A. Gamma delta T lymphocytes regulate the induction and maintenance of oral tolerance. J Immunol. 1997 Apr 15;158(8):3610–3618. [PubMed] [Google Scholar]
- King D. P., Hyde D. M., Jackson K. A., Novosad D. M., Ellis T. N., Putney L., Stovall M. Y., Van Winkle L. S., Beaman B. L., Ferrick D. A. Cutting edge: protective response to pulmonary injury requires gamma delta T lymphocytes. J Immunol. 1999 May 1;162(9):5033–5036. [PubMed] [Google Scholar]
- Komano H., Fujiura Y., Kawaguchi M., Matsumoto S., Hashimoto Y., Obana S., Mombaerts P., Tonegawa S., Yamamoto H., Itohara S. Homeostatic regulation of intestinal epithelia by intraepithelial gamma delta T cells. Proc Natl Acad Sci U S A. 1995 Jun 20;92(13):6147–6151. doi: 10.1073/pnas.92.13.6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahn M., Kanehiro A., Takeda K., Joetham A., Schwarze J., Köhler G., O'Brien R., Gelfand E. W., Born W., Kanehio A. Negative regulation of airway responsiveness that is dependent on gammadelta T cells and independent of alphabeta T cells. Nat Med. 1999 Oct;5(10):1150–1156. doi: 10.1038/13476. [DOI] [PubMed] [Google Scholar]
- Lee H. B., Kim J. H., Yim C. Y., Kim D. G., Ahn D. S. Differences in immunophenotyping of mucosal lymphocytes between ulcerative colitis and Crohn's disease. Korean J Intern Med. 1997 Jan;12(1):7–15. doi: 10.3904/kjim.1997.12.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVay L. D., Li B., Biancaniello R., Creighton M. A., Bachwich D., Lichtenstein G., Rombeau J. L., Carding S. R. Changes in human mucosal gamma delta T cell repertoire and function associated with the disease process in inflammatory bowel disease. Mol Med. 1997 Mar;3(3):183–203. [PMC free article] [PubMed] [Google Scholar]
- Mizoguchi A., Mizoguchi E., Chiba C., Spiekermann G. M., Tonegawa S., Nagler-Anderson C., Bhan A. K. Cytokine imbalance and autoantibody production in T cell receptor-alpha mutant mice with inflammatory bowel disease. J Exp Med. 1996 Mar 1;183(3):847–856. doi: 10.1084/jem.183.3.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris G. P., Beck P. L., Herridge M. S., Depew W. T., Szewczuk M. R., Wallace J. L. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology. 1989 Mar;96(3):795–803. [PubMed] [Google Scholar]
- Neurath M. F., Fuss I., Kelsall B. L., Stüber E., Strober W. Antibodies to interleukin 12 abrogate established experimental colitis in mice. J Exp Med. 1995 Nov 1;182(5):1281–1290. doi: 10.1084/jem.182.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto S., Watanabe M., Yamazaki M., Yajima T., Hayashi T., Ishii H., Mukai M., Yamada T., Watanabe N., Jameson B. A. A synthetic mimetic of CD4 is able to suppress disease in a rodent model of immune colitis. Eur J Immunol. 1999 Jan;29(1):355–366. doi: 10.1002/(SICI)1521-4141(199901)29:01<355::AID-IMMU355>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Pelegrí C., Kühnlein P., Buchner E., Schmidt C. B., Franch A., Castell M., Hünig T., Emmrich F., Kinne R. W. Depletion of gamma/delta T cells does not prevent or ameliorate, but rather aggravates, rat adjuvant arthritis. Arthritis Rheum. 1996 Feb;39(2):204–215. doi: 10.1002/art.1780390206. [DOI] [PubMed] [Google Scholar]
- Pospai D., René E., Fiasse R., Farahat K., Beaugery L., Lammens P., Reimund C., Duclos B., Le Quintrec Y., Vandercam B. Crohn's disease stable remission after human immunodeficiency virus infection. Dig Dis Sci. 1998 Feb;43(2):412–419. doi: 10.1023/a:1018883112012. [DOI] [PubMed] [Google Scholar]
- Roberts S. J., Smith A. L., West A. B., Wen L., Findly R. C., Owen M. J., Hayday A. C. T-cell alpha beta + and gamma delta + deficient mice display abnormal but distinct phenotypes toward a natural, widespread infection of the intestinal epithelium. Proc Natl Acad Sci U S A. 1996 Oct 15;93(21):11774–11779. doi: 10.1073/pnas.93.21.11774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shizuru J. A., Seydel K. B., Flavin T. F., Wu A. P., Kong C. C., Hoyt E. G., Fujimoto N., Billingham M. E., Starnes V. A., Fathman C. G. Induction of donor-specific unresponsiveness to cardiac allografts in rats by pretransplant anti-CD4 monoclonal antibody therapy. Transplantation. 1990 Sep;50(3):366–373. doi: 10.1097/00007890-199009000-00002. [DOI] [PubMed] [Google Scholar]
- Simpson S. J., Holländer G. A., Mizoguchi E., Allen D., Bhan A. K., Wang B., Terhorst C. Expression of pro-inflammatory cytokines by TCR alpha beta+ and TCR gamma delta+ T cells in an experimental model of colitis. Eur J Immunol. 1997 Jan;27(1):17–25. doi: 10.1002/eji.1830270104. [DOI] [PubMed] [Google Scholar]
- Stuber E., Strober W., Neurath M. Blocking the CD40L-CD40 interaction in vivo specifically prevents the priming of T helper 1 cells through the inhibition of interleukin 12 secretion. J Exp Med. 1996 Feb 1;183(2):693–698. doi: 10.1084/jem.183.2.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K., Ota H., Sasagawa S., Sakatani T., Fujikura T. Assay method for myeloperoxidase in human polymorphonuclear leukocytes. Anal Biochem. 1983 Jul 15;132(2):345–352. doi: 10.1016/0003-2697(83)90019-2. [DOI] [PubMed] [Google Scholar]
- Szczepanik M., Anderson L. R., Ushio H., Ptak W., Owen M. J., Hayday A. C., Askenase P. W. Gamma delta T cells from tolerized alpha beta T cell receptor (TCR)-deficient mice inhibit contact sensitivity-effector T cells in vivo, and their interferon-gamma production in vitro. J Exp Med. 1996 Dec 1;184(6):2129–2139. doi: 10.1084/jem.184.6.2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich R., Schieferdecker H. L., Ziegler K., Riecken E. O., Zeitz M. gamma delta T cells in the human intestine express surface markers of activation and are preferentially located in the epithelium. Cell Immunol. 1990 Jul;128(2):619–627. doi: 10.1016/0008-8749(90)90053-t. [DOI] [PubMed] [Google Scholar]
- Watanabe M., Hosoda Y., Okamoto S., Yamazaki M., Inoue N., Ueno Y., Iwao Y., Ishii H., Watanabe N., Hamada Y. CD45RChighCD4+ intestinal mucosal lymphocytes infiltrating in the inflamed colonic mucosa of a novel rat colitis model induced by TNB immunization. Clin Immunol Immunopathol. 1998 Jul;88(1):46–55. doi: 10.1006/clin.1997.4508. [DOI] [PubMed] [Google Scholar]
- Zeeh J. M., Procaccino F., Hoffmann P., Aukerman S. L., McRoberts J. A., Soltani S., Pierce G. F., Lakshmanan J., Lacey D., Eysselein V. E. Keratinocyte growth factor ameliorates mucosal injury in an experimental model of colitis in rats. Gastroenterology. 1996 Apr;110(4):1077–1083. doi: 10.1053/gast.1996.v110.pm8612996. [DOI] [PubMed] [Google Scholar]