Abstract
BACKGROUND—Activated hepatic stellate cells (HSC) are central to the pathogenesis of liver fibrosis, both as a source of fibrillar collagens that characterise fibrosis and matrix degrading metalloproteinases and their tissue inhibitors, the TIMPs. AIMS—To test the hypothesis that HSC apoptosis is critical to recovery from biliary fibrosis and that soluble growth factors may regulate HSC survival and apoptosis. METHODS—Rats (n=15) were subjected to bile duct ligation for 21 days, after which biliodigestive anastomosis was undertaken (n=13). Livers were harvested at fixed time points of recovery for periods of up to 42 days. Numbers of activated HSCs were quantified after α smooth muscle actin staining and HSC apoptosis was detected by terminal UDP-nick end labelling (TUNEL) staining and quantified at each time point. HSC apoptosis was quantified in vitro in the presence or absence of insulin-like growth factor (IGF)-1, IGF-2, platelet derived growth factor (PDGF), and transforming growth factor β1 (TGF-β1). RESULTS—Following biliodigestive anastomosis after 21 days of bile duct ligation, rat liver demonstrated a progressive resolution of biliary fibrosis over 42 days, associated with a fivefold decrease in activated HSC determined by α smooth muscle actin staining. TUNEL staining indicated that loss of activated HSC resulted from an increase in the rate of apoptosis during the first two days post biliodigestive anastomosis. Serum deprivation and culture in the presence of 50 µM cycloheximide was associated with an increase in HSC apoptosis which was significantly inhibited by addition of 10 ng/ml and 100 ng/ml IGF-1, respectively (0.05>p, n=5). In contrast, 1 and 10 ng/ml of TGF-β1 caused a significant increase in HSC apoptosis compared with serum free controls (p<0.05, n=4). PDGF and IGF-2 were neutral with respect to their effect on HSC apoptosis. CONCLUSION—HSC apoptosis plays a critical role in the spontaneous recovery from biliary fibrosis. Both survival and apoptosis of HSC are regulated by growth factors expressed during fibrotic liver injury. Keywords: hepatic stellate cells; apoptosis; hepatic fibrosis; insulin-like growth factor; transforming growth factor β1
Full Text
The Full Text of this article is available as a PDF (283.6 KB).
Figure 1 .

Histological analysis at peak fibrosis, 21 days after bile duct ligation (BDL). Livers harvested from animals 21 days after BDL were stained with reticulin (A, ×10) and Sirius red (B, ×20). A highly distinct pattern of matrix and collagen is apparent in the expanded portal tracts. This neomatrix extends in a septate manner into the parenchyma, in places linking portal tracts, and beginning to distort the liver architecture. Histological analysis of animals 42 days after biliary-jejunal anastomosis: following biliary-jejunal anastomosis there was a progressive diminution in the stainable collagen and matrix both within the parenchyma and adjacent to the bile ducts. At 42 days post anastomosis, reticulin staining (C, ×10) and Sirius red staining (D, ×10) demonstrated that considerable matrix remodelling had taken place with a return to near normal patterns of collagen and matrix.
Figure 2 .

Alpha smooth muscle actin (α-SMA) staining of each liver sample was used to demonstrate activated hepatic stellate cells (HSCs). A progressive diminution of the number of positive cells was observed when fibrotic liver, 21 days after bile duct ligation (BDL), was compared with liver samples harvested after biliary-jejunal anastomosis. Representative examples are shown of α-SMA staining at peak fibrosis (21 days after BDL) (A) and after 42 days of biliary-jejunal anastomosis (B) (each at ×10). Activated HSCs are observed within and around the periportal neomatrix and around the developing fibrotic septae (A). After 42 days of recovery, residual activated HSC are present in the periportal region but are considerably diminished in number. (C) Overall results of quantification of numbers of activated HSC defined by α-SMA expression. There is progressive diminution in activated HSCs following biliary-jejunal anastomosis, but even after 42 days the number is still raised above that observed in sham operated controls.
Figure 3 .

Two examples of apoptotic hepatic stellate cells (HSCs) demonstrated by dual terminal UDP-nick end labelling (TUNEL) and α smooth muscle actin (α-SMA) staining. Liver sections at two days after biliary-jejunal anastomosis were dual stained with α-SMA and TUNEL. Non-parenchymal TUNEL positive cell nuclei (red) demonstrating the characteristic condensation and halo are observed within the α-SMA positive cells (blue), indicating that the apoptotic nuclei lie within activated HSCs (×40).
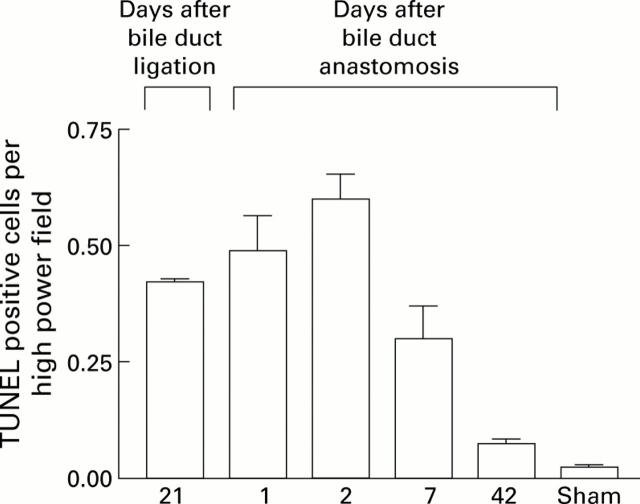
Figure 4 .
Quantification of non-parenchymal cell apoptosis during recovery from bile duct ligation (BDL). Terminal UDP-nick end labelling (TUNEL) positive non-parenchymal cell apoptotic bodies in a distribution consistent with activated hepatic stellate cells (HSCs) were quantified as described in the methods both before and after biliary-jejunal anastomosis. The results indicated that the first two days following biliary reanastomosis are associated with an increase in apoptosis, which coincides with the observed diminution in activated HSCs.
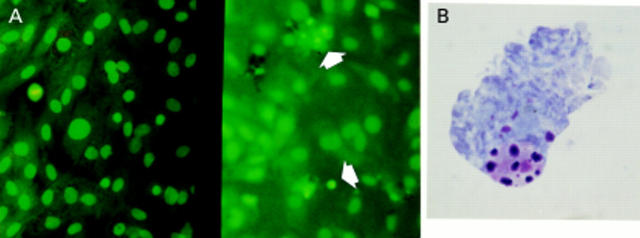
Figure 5 .
Acridine orange staining illustrating normal and apoptotic activated hepatic stellate cells (HSCs). (A) Representative fields from passaged HSC incubated for four days in media with 16% serum (left) and in serum free conditions (right). Normal HSC have large pale nuclei and orange tinged cytoplasm (the result of RNA staining). Apoptotic HSC are distinct with brightly condensed chromatin with shrunken or absent cytoplasm; two of several examples are arrowed. Apoptotic cells lift off from the monolayer, therefore to focus on these the monolayer falls below the plane of focus. Mitotic figures are also identifiable with this method (left field). Giemsa stain of cytospin preparation of condensed HSC: after exposure to conditions of absolute serum deprivation as described in the methods, the condensed rounded up cells on the monolayer surface, corresponding to the cells counted after acridine orange staining, were harvested by gentle washing and prepared as a cytospin, before being Giemsa stained. The cells demonstrated clear evidence of chromatin condensation and fragmentation, and blebbing; a representative example is given in (B). DNA analysis of the condensed and rounded up cells was undertaken as described in the methods and demonstrates the characteristic laddering associated with apoptosis (C).
Figure 6 .
Effect of prolonged serum deprivation on hepatic stellate cell (HSC) apoptosis. Rat HSCs were washed three times and incubated for 6, 24, and 96 hours in media with no additives (0% fetal calf serum (FCS)) or returned to media with 16% FCS. After incubation, the number of apoptotic cells was counted and expressed as a percentage of the total cell number per field (mean (SEM), n=7; *p<0.05, **p<0.01 for 0% FCS v 16% FCS at the same time point by paired t test).
Figure 7 .
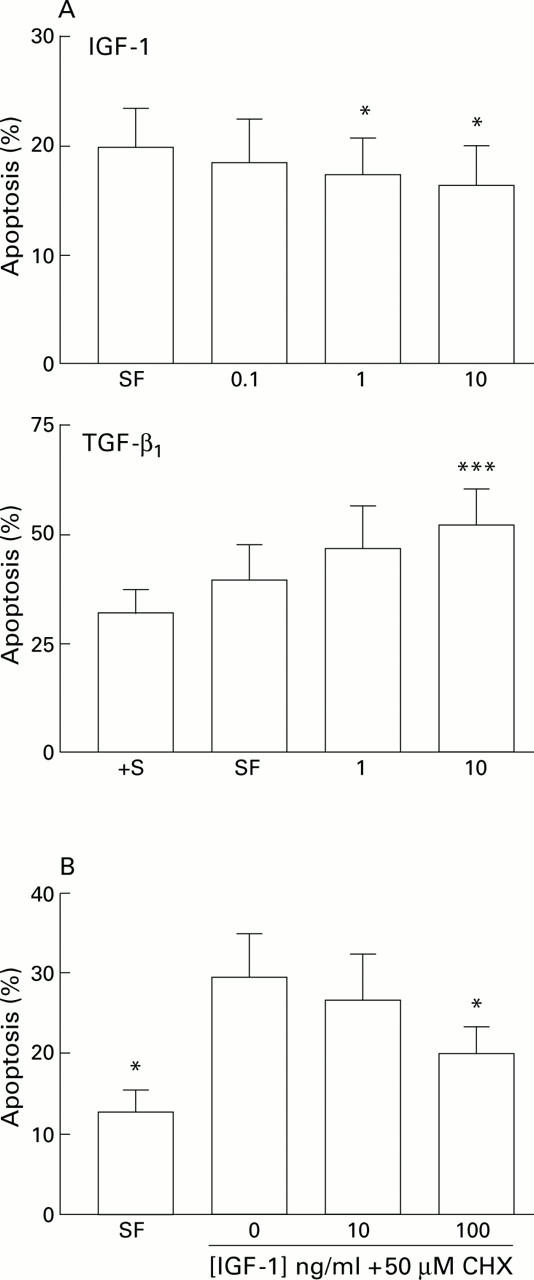
(A) Effect of specific growth factors on hepatic stellate cell (HSC) apoptosis. Effect of insulin-like growth factor 1 (IGF-1) and transforming growth factor β1 (TGF-β1) on serum deprivation induced apoptosis of rat HSCs. For each concentration of growth factor (x axis, ng/ml) the percentage of cells demonstrating an apoptotic morphology after staining with acridine orange is expressed as mean (SEM). For the IGF-1 experiments, counting was undertaken after 24 hours (n=5, *p<0.05 v no additives by paired t test). For the TGF-β1 experiments, counting was undertaken after six hours (***p<0.001 by paired t test, n=4). †S, serum containing media. (B) Effects of IGF-1 on cycloheximide induced apoptosis of rat HSCs. Washed rat HSC cultures were incubated for six hours in media with no additives, or media supplemented with 50 µM cycloheximide (CHX) with or without IGF-1. The percentage of cells undergoing apoptosis detected by acridine orange staining is given as mean (SEM) (n=4). *p<0.05 for IGF-1 treated cultures v cycloheximide alone by paired t test. SF, serum free.
Figure 8 .
(A) Effect of growth factors on 3H-thymidine incorporation. After washing, rat hepatic stellate cells (HSCs) were cultured in DMEM with no additives (serum free (SF)), 16% fetal calf serum (FCS) (+S, positive control), or specified growth factors for 24 hours, and the rate of 3H-thymidine incorporated was determined as described. Results are expressed relative to control cultures (no additives, SF) which were given the arbitrary value of 100% (n=4, *p<0.05 relative to 16% FCS containing cultures by paired t test). (B) Effect of growth factors on total DNA of cultures of rat HSCs. Washed cultures of rat HSCs were incubated for 24 hours in DMEM with no additives (SF) or supplemented with 16% FCS (+S, positive control) or growth factors as shown and the total DNA concentration of each cell suspension was determined as described in the methods. Results are expressed relative to control cultures (no additives, SF) which were given the arbitrary value of 100% (n=4, *p<0.05 relative to 16% FCS containing cultures by paired t test). (C, D) Effect of transforming growth factor β1 (TGF-β1) on HSC proliferation and total DNA content, respectively, were analysed as described and are represented relative to control cultures (no additives, SF) which have been given the arbitrary value of 100%.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdel-Aziz G., Lebeau G., Rescan P. Y., Clément B., Rissel M., Deugnier Y., Campion J. P., Guillouzo A. Reversibility of hepatic fibrosis in experimentally induced cholestasis in rat. Am J Pathol. 1990 Dec;137(6):1333–1342. [PMC free article] [PubMed] [Google Scholar]
- Alcolado R., Arthur M. J., Iredale J. P. Pathogenesis of liver fibrosis. Clin Sci (Lond) 1997 Feb;92(2):103–112. doi: 10.1042/cs0920103. [DOI] [PubMed] [Google Scholar]
- Arthur M. J., Friedman S. L., Roll F. J., Bissell D. M. Lipocytes from normal rat liver release a neutral metalloproteinase that degrades basement membrane (type IV) collagen. J Clin Invest. 1989 Oct;84(4):1076–1085. doi: 10.1172/JCI114270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker A. J., Mooney A., Hughes J., Lombardi D., Johnson R. J., Savill J. Mesangial cell apoptosis: the major mechanism for resolution of glomerular hypercellularity in experimental mesangial proliferative nephritis. J Clin Invest. 1994 Nov;94(5):2105–2116. doi: 10.1172/JCI117565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenzel A., Gressner A. M. Characterization of insulin-like growth factor (IGF)-I-receptor binding sites during in vitro transformation of rat hepatic stellate cells to myofibroblasts. Eur J Clin Chem Clin Biochem. 1996 May;34(5):401–409. doi: 10.1515/cclm.1996.34.5.401. [DOI] [PubMed] [Google Scholar]
- Evan G. I., Wyllie A. H., Gilbert C. S., Littlewood T. D., Land H., Brooks M., Waters C. M., Penn L. Z., Hancock D. C. Induction of apoptosis in fibroblasts by c-myc protein. Cell. 1992 Apr 3;69(1):119–128. doi: 10.1016/0092-8674(92)90123-t. [DOI] [PubMed] [Google Scholar]
- Fanidi A., Harrington E. A., Evan G. I. Cooperative interaction between c-myc and bcl-2 proto-oncogenes. Nature. 1992 Oct 8;359(6395):554–556. doi: 10.1038/359554a0. [DOI] [PubMed] [Google Scholar]
- Friedman S. L., Roll F. J., Boyles J., Bissell D. M. Hepatic lipocytes: the principal collagen-producing cells of normal rat liver. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8681–8685. doi: 10.1073/pnas.82.24.8681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman S. L. Seminars in medicine of the Beth Israel Hospital, Boston. The cellular basis of hepatic fibrosis. Mechanisms and treatment strategies. N Engl J Med. 1993 Jun 24;328(25):1828–1835. doi: 10.1056/NEJM199306243282508. [DOI] [PubMed] [Google Scholar]
- Gong W., Pecci A., Roth S., Lahme B., Beato M., Gressner A. M. Transformation-dependent susceptibility of rat hepatic stellate cells to apoptosis induced by soluble Fas ligand. Hepatology. 1998 Aug;28(2):492–502. doi: 10.1002/hep.510280229. [DOI] [PubMed] [Google Scholar]
- Gressner A. M. The cell biology of liver fibrogenesis - an imbalance of proliferation, growth arrest and apoptosis of myofibroblasts. Cell Tissue Res. 1998 Jun;292(3):447–452. doi: 10.1007/s004410051073. [DOI] [PubMed] [Google Scholar]
- Iredale J. P., Benyon R. C., Arthur M. J., Ferris W. F., Alcolado R., Winwood P. J., Clark N., Murphy G. Tissue inhibitor of metalloproteinase-1 messenger RNA expression is enhanced relative to interstitial collagenase messenger RNA in experimental liver injury and fibrosis. Hepatology. 1996 Jul;24(1):176–184. doi: 10.1002/hep.510240129. [DOI] [PubMed] [Google Scholar]
- Iredale J. P., Benyon R. C., Pickering J., McCullen M., Northrop M., Pawley S., Hovell C., Arthur M. J. Mechanisms of spontaneous resolution of rat liver fibrosis. Hepatic stellate cell apoptosis and reduced hepatic expression of metalloproteinase inhibitors. J Clin Invest. 1998 Aug 1;102(3):538–549. doi: 10.1172/JCI1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. R., Upadhyay S., Li G., Palmer K. C., Deuel T. F. Platelet-derived growth factor induces apoptosis in growth-arrested murine fibroblasts. Proc Natl Acad Sci U S A. 1995 Oct 10;92(21):9500–9504. doi: 10.1073/pnas.92.21.9500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher J. J., McGuire R. F. Extracellular matrix gene expression increases preferentially in rat lipocytes and sinusoidal endothelial cells during hepatic fibrosis in vivo. J Clin Invest. 1990 Nov;86(5):1641–1648. doi: 10.1172/JCI114886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinzani M., Abboud H. E., Aron D. C. Secretion of insulin-like growth factor-I and binding proteins by rat liver fat-storing cells: regulatory role of platelet-derived growth factor. Endocrinology. 1990 Nov;127(5):2343–2349. doi: 10.1210/endo-127-5-2343. [DOI] [PubMed] [Google Scholar]
- Pinzani M., Gesualdo L., Sabbah G. M., Abboud H. E. Effects of platelet-derived growth factor and other polypeptide mitogens on DNA synthesis and growth of cultured rat liver fat-storing cells. J Clin Invest. 1989 Dec;84(6):1786–1793. doi: 10.1172/JCI114363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnicoff M., Abraham D., Yutanawiboonchai W., Rotman H. L., Kajstura J., Rubin R., Zoltick P., Baserga R. The insulin-like growth factor I receptor protects tumor cells from apoptosis in vivo. Cancer Res. 1995 Jun 1;55(11):2463–2469. [PubMed] [Google Scholar]
- Resnicoff M., Burgaud J. L., Rotman H. L., Abraham D., Baserga R. Correlation between apoptosis, tumorigenesis, and levels of insulin-like growth factor I receptors. Cancer Res. 1995 Sep 1;55(17):3739–3741. [PubMed] [Google Scholar]
- Rockey D. C., Boyles J. K., Gabbiani G., Friedman S. L. Rat hepatic lipocytes express smooth muscle actin upon activation in vivo and in culture. J Submicrosc Cytol Pathol. 1992 Apr;24(2):193–203. [PubMed] [Google Scholar]
- Rojkind M., Dunn M. A. Hepatic fibrosis. Gastroenterology. 1979 Apr;76(4):849–863. [PubMed] [Google Scholar]
- Romano G., Prisco M., Zanocco-Marani T., Peruzzi F., Valentinis B., Baserga R. Dissociation between resistance to apoptosis and the transformed phenotype in IGF-I receptor signaling. J Cell Biochem. 1999 Feb 1;72(2):294–310. doi: 10.1002/(sici)1097-4644(19990201)72:2<294::aid-jcb14>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Saile B., Knittel T., Matthes N., Schott P., Ramadori G. CD95/CD95L-mediated apoptosis of the hepatic stellate cell. A mechanism terminating uncontrolled hepatic stellate cell proliferation during hepatic tissue repair. Am J Pathol. 1997 Nov;151(5):1265–1272. [PMC free article] [PubMed] [Google Scholar]
- Scharf J. G., Schmidt-Sandte W., Pahernik S. A., Ramadori G., Braulke T., Hartmann H. Characterization of the insulin-like growth factor axis in a human hepatoma cell line (PLC). Carcinogenesis. 1998 Dec;19(12):2121–2128. doi: 10.1093/carcin/19.12.2121. [DOI] [PubMed] [Google Scholar]
- Stähelin B. J., Marti U., Solioz M., Zimmermann H., Reichen J. False positive staining in the TUNEL assay to detect apoptosis in liver and intestine is caused by endogenous nucleases and inhibited by diethyl pyrocarbonate. Mol Pathol. 1998 Aug;51(4):204–208. doi: 10.1136/mp.51.4.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stähelin B. J., Marti U., Zimmermann H., Reichen J. The interaction of Bcl-2 and Bax regulates apoptosis in biliary epithelial cells of rats with obstructive jaundice. Virchows Arch. 1999 Apr;434(4):333–339. doi: 10.1007/s004280050349. [DOI] [PubMed] [Google Scholar]
- Valentinis B., Reiss K., Baserga R. Insulin-like growth factor-I-mediated survival from anoikis: role of cell aggregation and focal adhesion kinase. J Cell Physiol. 1998 Sep;176(3):648–657. doi: 10.1002/(SICI)1097-4652(199809)176:3<648::AID-JCP22>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Vyas S. K., Leyland H., Gentry J., Arthur M. J. Rat hepatic lipocytes synthesize and secrete transin (stromelysin) in early primary culture. Gastroenterology. 1995 Sep;109(3):889–898. doi: 10.1016/0016-5085(95)90399-2. [DOI] [PubMed] [Google Scholar]
- Weil M., Jacobson M. D., Coles H. S., Davies T. J., Gardner R. L., Raff K. D., Raff M. C. Constitutive expression of the machinery for programmed cell death. J Cell Biol. 1996 Jun;133(5):1053–1059. doi: 10.1083/jcb.133.5.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]