Abstract
BACKGROUND AND AIMS—An association between the allele 2 of the interleukin 1 receptor antagonist gene variable number tandem repeats polymorphism in intron 2 and ulcerative colitis was first reported in 1994. Subsequent studies in Caucasian Northern European patients have not confirmed this, although trends towards an association were observed. The lack of statistical significance could reflect inadequate power. In this study the association was reassessed in a large independent set of well characterised Caucasian patients and a meta-analysis of reported patient series was performed. PATIENTS AND METHODS—A total of 320 patients with endoscopically and histologically confirmed ulcerative colitis (124 pancolitis, 196 left sided and distal disease) and 827 ethnically matched controls were genotyped at polymorphic sites in the interleukin 1 receptor antagonist gene. Carriage rates were compared using χ2 statistics. A meta-analysis of this and seven previous studies in North European Caucasian patients was performed using the Mantel-Haenszel χ2 test. RESULTS—Patients had a significantly increased carriage rate of allele 2 compared with controls (52% v 45%; odds ratio 1.3 (95% confidence interval (CI) 1.01-1.7); p=0.04). The allele 2 carriage rate was highest in extensive colitis (carriage rate 56%; odds ratio 1.5 (95% CI 1.1-2.3) p=0.02) and in individuals who had undergone colectomy (carriage rate 55%; odds ratio 1.5 (95% CI 0.95-2.4); p=0.08). Meta-analysis of all eight studies showed a significant association between carriage of allele 2 and ulcerative colitis (odds ratio 1.23 (95% CI 1.04-1.45); p=0.01). CONCLUSIONS—The association of the interleukin 1 receptor antagonist gene polymorphism with ulcerative colitis is confirmed. The association is minor and confers only a small risk to an individual but will contribute a high attributable risk in a population due to the high allelic frequency. Accurate phenotypic characterisation defines more homogeneous subsets of patients, such as those with extensive disease, in whom the association is greater. Keywords: ulcerative colitis; cytokine gene polymorphisms; interleukin 1 receptor antagonist; interleukin 1; inflammatory bowel disease; genetics
Full Text
The Full Text of this article is available as a PDF (154.1 KB).
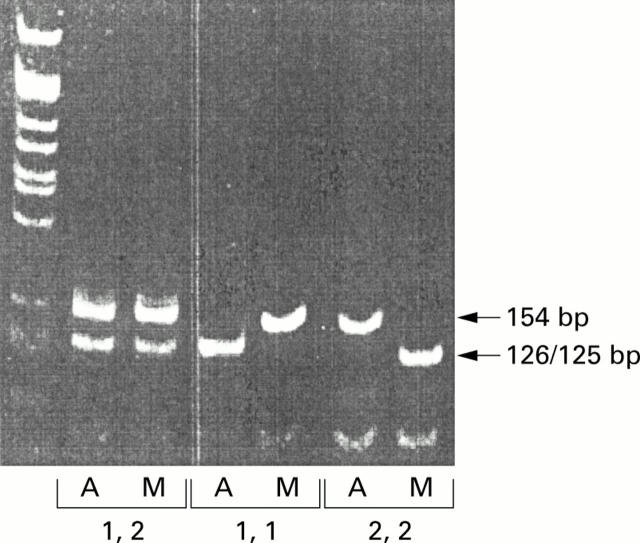
Figure 1 .
Interleukin 1 receptor antagonist gene (IL-1RN) +2018 exon 2 single nucleotide polymorphism genotyping using restriction enzyme digestion of amplified polymerase chain reaction (PCR) products. Lane 1 contains a standard DNA ladder. Lanes 2-7 contain DNA from three individuals. Amplified PCR products from each individual were divided into two aliquots and separately digested with AluI or MspI. PCR products were size fractionated by 9% polyacrylamide gel electrophoresis and visualised under ultraviolet light after staining with ethidium bromide. The two enzymes cut the two alleles differently. AluI cuts the 154 base pair fragment to produce 126 and 28 base pair fragments in allele 1 while it does not digest allele 2 (154 base pairs). MspI digestion produces 125 and 29 base pair fragments in allele 2 but does not digest allele 1 (154 base pairs). Hence the two reactions (separated side by side on a gel) will give inverted patterns of digestion for homozygous individuals (lanes 4/5 and 6/7), and identical patterns in heterozygotes (lanes 2/3).
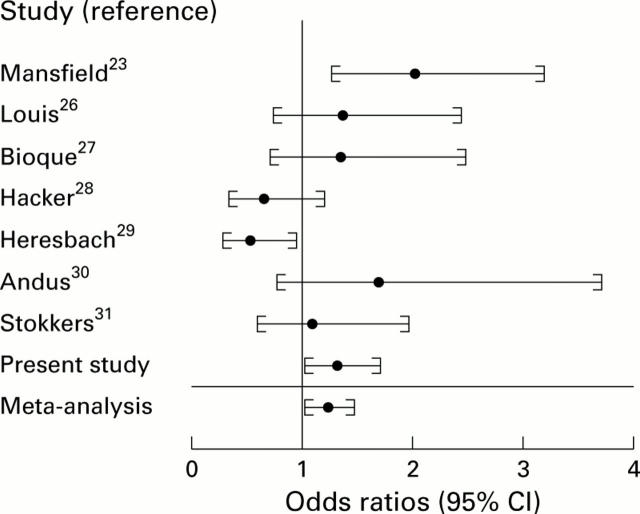
Figure 2 .
Graphic display of individual and summary odds ratios with 95% confidence intervals (CI) for the seven previously published studies of the interleukin 1 receptor antagonist gene (IL-1RN) polymorphism in ulcerative colitis, the present study, and the meta-analysis. Studies are shown as first author with the reference number.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andus T., Daig R., Vogl D., Aschenbrenner E., Lock G., Hollerbach S., Köllinger M., Schölmerich J., Gross V. Imbalance of the interleukin 1 system in colonic mucosa--association with intestinal inflammation and interleukin 1 receptor antagonist [corrected] genotype 2. Gut. 1997 Nov;41(5):651–657. doi: 10.1136/gut.41.5.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arend W. P. Interleukin-1 receptor antagonist. Adv Immunol. 1993;54:167–227. doi: 10.1016/s0065-2776(08)60535-0. [DOI] [PubMed] [Google Scholar]
- Bioque G., Bouma G., Crusius J. B., Koutroubakis I., Kostense P. J., Meuwissen S. G., Peña A. S. Evidence of genetic heterogeneity in IBD: 1. The interleukin-1 receptor antagonist in the predisposition to suffer from ulcerative colitis. Eur J Gastroenterol Hepatol. 1996 Feb;8(2):105–110. [PubMed] [Google Scholar]
- Brett P. M., Yasuda N., Yiannakou J. Y., Herbst F., Ellis H. J., Vaughan R., Nicholls R. J., Ciclitira P. J. Genetic and immunological markers in pouchitis. Eur J Gastroenterol Hepatol. 1996 Oct;8(10):951–955. doi: 10.1097/00042737-199610000-00003. [DOI] [PubMed] [Google Scholar]
- Casini-Raggi V., Kam L., Chong Y. J., Fiocchi C., Pizarro T. T., Cominelli F. Mucosal imbalance of IL-1 and IL-1 receptor antagonist in inflammatory bowel disease. A novel mechanism of chronic intestinal inflammation. J Immunol. 1995 Mar 1;154(5):2434–2440. [PubMed] [Google Scholar]
- Clay F. E., Tarlow J. K., Cork M. J., Cox A., Nicklin M. J., Duff G. W. Novel interleukin-1 receptor antagonist exon polymorphisms and their use in allele-specific mRNA assessment. Hum Genet. 1996 Jun;97(6):723–726. doi: 10.1007/BF02346180. [DOI] [PubMed] [Google Scholar]
- Cominelli F., Nast C. C., Clark B. D., Schindler R., Lierena R., Eysselein V. E., Thompson R. C., Dinarello C. A. Interleukin 1 (IL-1) gene expression, synthesis, and effect of specific IL-1 receptor blockade in rabbit immune complex colitis. J Clin Invest. 1990 Sep;86(3):972–980. doi: 10.1172/JCI114799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cominelli F., Nast C. C., Duchini A., Lee M. Recombinant interleukin-1 receptor antagonist blocks the proinflammatory activity of endogenous interleukin-1 in rabbit immune colitis. Gastroenterology. 1992 Jul;103(1):65–71. doi: 10.1016/0016-5085(92)91096-m. [DOI] [PubMed] [Google Scholar]
- Cox A., Camp N. J., Nicklin M. J., di Giovine F. S., Duff G. W. An analysis of linkage disequilibrium in the interleukin-1 gene cluster, using a novel grouping method for multiallelic markers. Am J Hum Genet. 1998 May;62(5):1180–1188. doi: 10.1086/301817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A., Wolff S. M. The role of interleukin-1 in disease. N Engl J Med. 1993 Jan 14;328(2):106–113. doi: 10.1056/NEJM199301143280207. [DOI] [PubMed] [Google Scholar]
- Farmer R. G., Easley K. A., Rankin G. B. Clinical patterns, natural history, and progression of ulcerative colitis. A long-term follow-up of 1116 patients. Dig Dis Sci. 1993 Jun;38(6):1137–1146. doi: 10.1007/BF01295733. [DOI] [PubMed] [Google Scholar]
- Ferretti M., Casini-Raggi V., Pizarro T. T., Eisenberg S. P., Nast C. C., Cominelli F. Neutralization of endogenous IL-1 receptor antagonist exacerbates and prolongs inflammation in rabbit immune colitis. J Clin Invest. 1994 Jul;94(1):449–453. doi: 10.1172/JCI117345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guasch J. F., Bertina R. M., Reitsma P. H. Five novel intragenic dimorphisms in the human interleukin-1 genes combine to high informativity. Cytokine. 1996 Aug;8(8):598–602. doi: 10.1006/cyto.1996.0080. [DOI] [PubMed] [Google Scholar]
- Hacker U. T., Gomolka M., Keller E., Eigler A., Folwaczny C., Fricke H., Albert E., Loeschke K., Endres S. Lack of association between an interleukin-1 receptor antagonist gene polymorphism and ulcerative colitis. Gut. 1997 May;40(5):623–627. doi: 10.1136/gut.40.5.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heresbach D., Alizadeh M., Dabadie A., Le Berre N., Colombel J. F., Yaouanq J., Bretagne J. F., Semana G. Significance of interleukin-1beta and interleukin-1 receptor antagonist genetic polymorphism in inflammatory bowel diseases. Am J Gastroenterol. 1997 Jul;92(7):1164–1169. [PubMed] [Google Scholar]
- Hirsch E., Irikura V. M., Paul S. M., Hirsh D. Functions of interleukin 1 receptor antagonist in gene knockout and overproducing mice. Proc Natl Acad Sci U S A. 1996 Oct 1;93(20):11008–11013. doi: 10.1073/pnas.93.20.11008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugot J. P., Laurent-Puig P., Gower-Rousseau C., Olson J. M., Lee J. C., Beaugerie L., Naom I., Dupas J. L., Van Gossum A., Orholm M. Mapping of a susceptibility locus for Crohn's disease on chromosome 16. Nature. 1996 Feb 29;379(6568):821–823. doi: 10.1038/379821a0. [DOI] [PubMed] [Google Scholar]
- Jenkins D., Balsitis M., Gallivan S., Dixon M. F., Gilmour H. M., Shepherd N. A., Theodossi A., Williams G. T. Guidelines for the initial biopsy diagnosis of suspected chronic idiopathic inflammatory bowel disease. The British Society of Gastroenterology Initiative. J Clin Pathol. 1997 Feb;50(2):93–105. doi: 10.1136/jcp.50.2.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligumsky M., Simon P. L., Karmeli F., Rachmilewitz D. Role of interleukin 1 in inflammatory bowel disease--enhanced production during active disease. Gut. 1990 Jun;31(6):686–689. doi: 10.1136/gut.31.6.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis E., Satsangi J., Roussomoustakaki M., Parkes M., Fanning G., Welsh K., Jewell D. Cytokine gene polymorphisms in inflammatory bowel disease. Gut. 1996 Nov;39(5):705–710. doi: 10.1136/gut.39.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANTEL N., HAENSZEL W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959 Apr;22(4):719–748. [PubMed] [Google Scholar]
- Mahida Y. R., Wu K., Jewell D. P. Enhanced production of interleukin 1-beta by mononuclear cells isolated from mucosa with active ulcerative colitis of Crohn's disease. Gut. 1989 Jun;30(6):835–838. doi: 10.1136/gut.30.6.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansfield J. C., Holden H., Tarlow J. K., Di Giovine F. S., McDowell T. L., Wilson A. G., Holdsworth C. D., Duff G. W. Novel genetic association between ulcerative colitis and the anti-inflammatory cytokine interleukin-1 receptor antagonist. Gastroenterology. 1994 Mar;106(3):637–642. doi: 10.1016/0016-5085(94)90696-3. [DOI] [PubMed] [Google Scholar]
- Mantel N., Fleiss J. L. Minimum expected cell size requirements for the Mantel-Haenszel one-degree-of-freedom chi-square test and a related rapid procedure. Am J Epidemiol. 1980 Jul;112(1):129–134. doi: 10.1093/oxfordjournals.aje.a112962. [DOI] [PubMed] [Google Scholar]
- McCall R. D., Haskill S., Zimmermann E. M., Lund P. K., Thompson R. C., Sartor R. B. Tissue interleukin 1 and interleukin-1 receptor antagonist expression in enterocolitis in resistant and susceptible rats. Gastroenterology. 1994 Apr;106(4):960–972. doi: 10.1016/0016-5085(94)90755-2. [DOI] [PubMed] [Google Scholar]
- Miller S. A., Dykes D. D., Polesky H. F. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988 Feb 11;16(3):1215–1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicklin M. J., Weith A., Duff G. W. A physical map of the region encompassing the human interleukin-1 alpha, interleukin-1 beta, and interleukin-1 receptor antagonist genes. Genomics. 1994 Jan 15;19(2):382–384. doi: 10.1006/geno.1994.1076. [DOI] [PubMed] [Google Scholar]
- Nishiyama T., Mitsuyama K., Toyonaga A., Sasaki E., Tanikawa K. Colonic mucosal interleukin 1 receptor antagonist in inflammatory bowel disease. Digestion. 1994;55(6):368–373. doi: 10.1159/000201167. [DOI] [PubMed] [Google Scholar]
- Niv Y., Bat L., Ron E., Theodor E. Change in the extent of colonic involvement in ulcerative colitis: a colonoscopic study. Am J Gastroenterol. 1987 Oct;82(10):1046–1051. [PubMed] [Google Scholar]
- Orholm M., Iselius L., Sørensen T. I., Munkholm P., Langholz E., Binder V. Investigation of inheritance of chronic inflammatory bowel diseases by complex segregation analysis. BMJ. 1993 Jan 2;306(6869):20–24. doi: 10.1136/bmj.306.6869.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orholm M., Munkholm P., Langholz E., Nielsen O. H., Sørensen T. I., Binder V. Familial occurrence of inflammatory bowel disease. N Engl J Med. 1991 Jan 10;324(2):84–88. doi: 10.1056/NEJM199101103240203. [DOI] [PubMed] [Google Scholar]
- Podolsky D. K. Inflammatory bowel disease (2) N Engl J Med. 1991 Oct 3;325(14):1008–1016. doi: 10.1056/NEJM199110033251406. [DOI] [PubMed] [Google Scholar]
- Risch N., Merikangas K. The future of genetic studies of complex human diseases. Science. 1996 Sep 13;273(5281):1516–1517. doi: 10.1126/science.273.5281.1516. [DOI] [PubMed] [Google Scholar]
- Roussomoustakaki M., Satsangi J., Welsh K., Louis E., Fanning G., Targan S., Landers C., Jewell D. P. Genetic markers may predict disease behavior in patients with ulcerative colitis. Gastroenterology. 1997 Jun;112(6):1845–1853. doi: 10.1053/gast.1997.v112.pm9178675. [DOI] [PubMed] [Google Scholar]
- Sartor R. B. Cytokines in intestinal inflammation: pathophysiological and clinical considerations. Gastroenterology. 1994 Feb;106(2):533–539. doi: 10.1016/0016-5085(94)90614-9. [DOI] [PubMed] [Google Scholar]
- Sasieni P. D. From genotypes to genes: doubling the sample size. Biometrics. 1997 Dec;53(4):1253–1261. [PubMed] [Google Scholar]
- Satsangi J., Jewell D. P., Bell J. I. The genetics of inflammatory bowel disease. Gut. 1997 May;40(5):572–574. doi: 10.1136/gut.40.5.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satsangi J., Parkes M., Louis E., Hashimoto L., Kato N., Welsh K., Terwilliger J. D., Lathrop G. M., Bell J. I., Jewell D. P. Two stage genome-wide search in inflammatory bowel disease provides evidence for susceptibility loci on chromosomes 3, 7 and 12. Nat Genet. 1996 Oct;14(2):199–202. doi: 10.1038/ng1096-199. [DOI] [PubMed] [Google Scholar]
- Smith D. E., Renshaw B. R., Ketchem R. R., Kubin M., Garka K. E., Sims J. E. Four new members expand the interleukin-1 superfamily. J Biol Chem. 2000 Jan 14;275(2):1169–1175. doi: 10.1074/jbc.275.2.1169. [DOI] [PubMed] [Google Scholar]
- Spielman R. S., Ewens W. J. The TDT and other family-based tests for linkage disequilibrium and association. Am J Hum Genet. 1996 Nov;59(5):983–989. [PMC free article] [PubMed] [Google Scholar]
- Stokkers P. C., van Aken B. E., Basoski N., Reitsma P. H., Tytgat G. N., van Deventer S. J. Five genetic markers in the interleukin 1 family in relation to inflammatory bowel disease. Gut. 1998 Jul;43(1):33–39. doi: 10.1136/gut.43.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarlow J. K., Blakemore A. I., Lennard A., Solari R., Hughes H. N., Steinkasserer A., Duff G. W. Polymorphism in human IL-1 receptor antagonist gene intron 2 is caused by variable numbers of an 86-bp tandem repeat. Hum Genet. 1993 May;91(4):403–404. doi: 10.1007/BF00217368. [DOI] [PubMed] [Google Scholar]
- Tountas N. A., Casini-Raggi V., Yang H., Di Giovine F. S., Vecchi M., Kam L., Melani L., Pizarro T. T., Rotter J. I., Cominelli F. Functional and ethnic association of allele 2 of the interleukin-1 receptor antagonist gene in ulcerative colitis. Gastroenterology. 1999 Oct;117(4):806–813. doi: 10.1016/s0016-5085(99)70338-0. [DOI] [PubMed] [Google Scholar]
- Tysk C., Lindberg E., Järnerot G., Flodérus-Myrhed B. Ulcerative colitis and Crohn's disease in an unselected population of monozygotic and dizygotic twins. A study of heritability and the influence of smoking. Gut. 1988 Jul;29(7):990–996. doi: 10.1136/gut.29.7.990. [DOI] [PMC free article] [PubMed] [Google Scholar]