Abstract
BACKGROUND AND AIMS—Altered intestinal permeability is a key pathogenetic factor of idiopathic bowel inflammation. We investigated in the rat if changes in the composition of the bowel flora can alter colonic permeability. METHODS—A colonic segment was surgically excluded from faecal transit and brought out as a loop to the abdominal wall through two colostomies. The loop was used for colonisation with specific bacterial strains after eradication of the native flora with antibiotics. Lumen to blood clearance of dextran (molecular weight 70 000) and mannitol (molecular weight 182) was measured in rats recolonised with a single bacterial strain from rat colonic origin, and in control rats whose colonic loop was kept free of bacteria by antibiotics. Actual colonisation was confirmed by culture of segment effluents. RESULTS—Colonisation with Escherichia coli, Klebsiella pneumoniae, and Streptococcus viridans significantly increased lumen to blood clearance of mannitol. Colonisation with Lactobacillus brevis had the opposite effect and reduced permeability to mannitol. Bacteroides fragilis did not induce significant changes. Permeability to dextran was not altered by any of the strains tested. CONCLUSIONS—Certain commensal bacteria can modify colonic wall permeability to luminal substances. Keywords: bacteria; dextran; intestinal permeability; mannitol; rat
Full Text
The Full Text of this article is available as a PDF (171.5 KB).
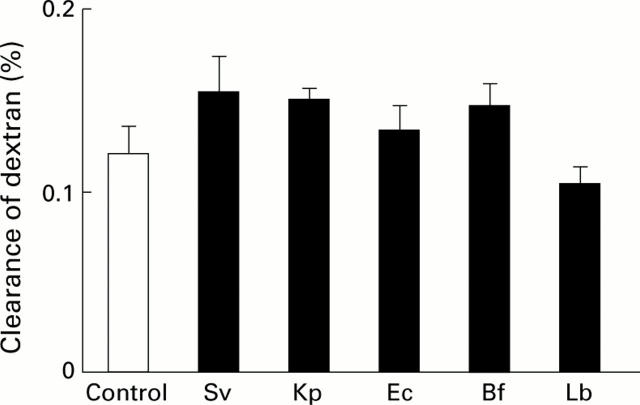
Figure 1 .
Lumen to blood clearance of dextran five hours after administration of the labelled probe into the excluded colonic segment of controls (n=10) and rats recolonised with a single bacterial strain (Sv, Streptococcus viridans, n=6; Kp, Klebsiella pneumoniae, n=6; Ec, Escherichia coli, n=7; Bf, Bacteroides fragilis, n=7; and Lb, Lactobacillus brevis, n=9). Clearance is expressed as a percentage of the administered dose that accumulated in the intravascular space and normalised by length of the excluded colonic segment. Values are mean (SEM). No significant differences were found.
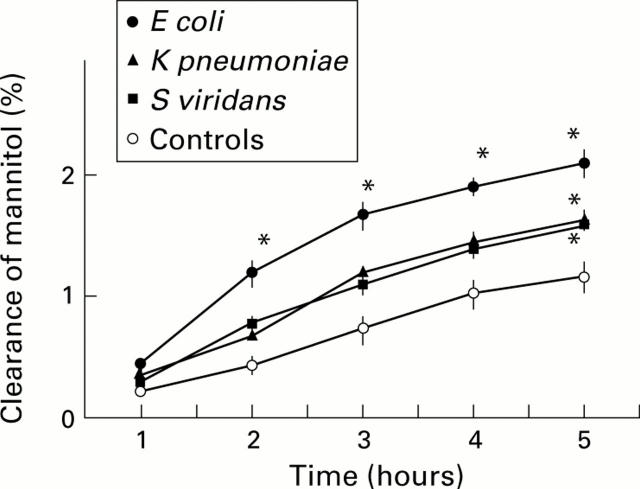
Figure 2 .
Lumen to blood clearance of mannitol in controls (n=10) and in rats recolonised with a single bacterial strain (Streptococcus viridans, n=6; Klebsiella pneumoniae, n=6; Escherichia coli, n=7). Clearance is expressed as a percentage of the administered dose that accumulated in the extracellular space and normalised by length of the colonic segment. Values are mean (SEM). *p<0.05 compared with controls at same time point.
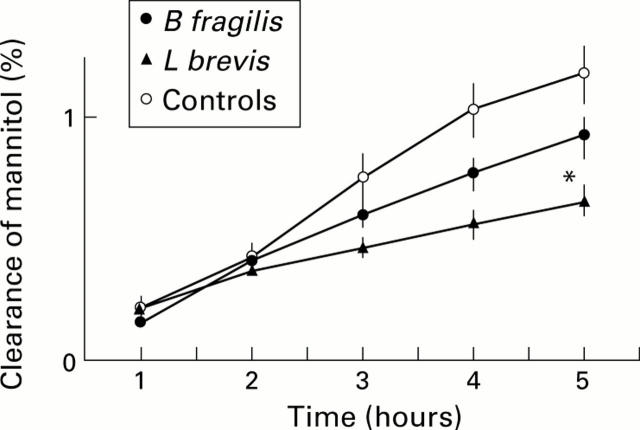
Figure 3 .
Lumen to blood clearance of mannitol in controls (n=10) and in rats recolonised with a single bacterial strain (Bacteroides fragilis, n=7; Lactobacillus brevis, n=9). Values are mean (SEM). *p<0.05 compared with controls at same time point.
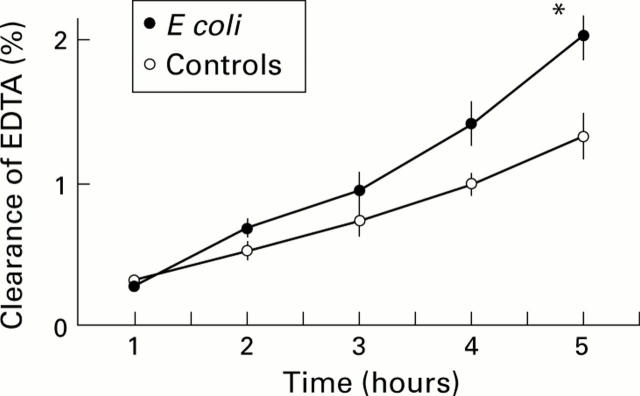
Figure 4 .
Lumen to blood clearance of 51Cr-EDTA in controls (n=8) and in rats recolonised with Escherichia coli (n=5). *p<0.05 compared with controls at same time point.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bjarnason I., MacPherson A., Hollander D. Intestinal permeability: an overview. Gastroenterology. 1995 May;108(5):1566–1581. doi: 10.1016/0016-5085(95)90708-4. [DOI] [PubMed] [Google Scholar]
- Brandtzaeg P., Halstensen T. S., Kett K., Krajci P., Kvale D., Rognum T. O., Scott H., Sollid L. M. Immunobiology and immunopathology of human gut mucosa: humoral immunity and intraepithelial lymphocytes. Gastroenterology. 1989 Dec;97(6):1562–1584. doi: 10.1016/0016-5085(89)90406-x. [DOI] [PubMed] [Google Scholar]
- García-Lafuente A., Antolín M., Guarner F., Crespo E., Salas A., Forcada P., Malagelada J. Derangement of mucosal barrier function by bacteria colonizing the rat colonic mucosa. Eur J Clin Invest. 1998 Dec;28(12):1019–1026. doi: 10.1046/j.1365-2362.1998.00405.x. [DOI] [PubMed] [Google Scholar]
- Hilsden R. J., Meddings J. B., Sutherland L. R. Intestinal permeability changes in response to acetylsalicylic acid in relatives of patients with Crohn's disease. Gastroenterology. 1996 May;110(5):1395–1403. doi: 10.1053/gast.1996.v110.pm8613043. [DOI] [PubMed] [Google Scholar]
- Isolauri E., Majamaa H., Arvola T., Rantala I., Virtanen E., Arvilommi H. Lactobacillus casei strain GG reverses increased intestinal permeability induced by cow milk in suckling rats. Gastroenterology. 1993 Dec;105(6):1643–1650. doi: 10.1016/0016-5085(93)91059-q. [DOI] [PubMed] [Google Scholar]
- Kindon H., Pothoulakis C., Thim L., Lynch-Devaney K., Podolsky D. K. Trefoil peptide protection of intestinal epithelial barrier function: cooperative interaction with mucin glycoprotein. Gastroenterology. 1995 Aug;109(2):516–523. doi: 10.1016/0016-5085(95)90340-2. [DOI] [PubMed] [Google Scholar]
- Lichtenberger L. M. The hydrophobic barrier properties of gastrointestinal mucus. Annu Rev Physiol. 1995;57:565–583. doi: 10.1146/annurev.ph.57.030195.003025. [DOI] [PubMed] [Google Scholar]
- Lugea A., Salas A., Casalot J., Guarner F., Malagelada J. R. Surface hydrophobicity of the rat colonic mucosa is a defensive barrier against macromolecules and toxins. Gut. 2000 Apr;46(4):515–521. doi: 10.1136/gut.46.4.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macpherson A., Khoo U. Y., Forgacs I., Philpott-Howard J., Bjarnason I. Mucosal antibodies in inflammatory bowel disease are directed against intestinal bacteria. Gut. 1996 Mar;38(3):365–375. doi: 10.1136/gut.38.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Y., Nobaek S., Kasravi B., Adawi D., Stenram U., Molin G., Jeppsson B. The effects of Lactobacillus strains and oat fiber on methotrexate-induced enterocolitis in rats. Gastroenterology. 1996 Aug;111(2):334–344. doi: 10.1053/gast.1996.v111.pm8690198. [DOI] [PubMed] [Google Scholar]
- Matsuo K., Ota H., Akamatsu T., Sugiyama A., Katsuyama T. Histochemistry of the surface mucous gel layer of the human colon. Gut. 1997 Jun;40(6):782–789. doi: 10.1136/gut.40.6.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris G. P., Beck P. L., Herridge M. S., Depew W. T., Szewczuk M. R., Wallace J. L. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology. 1989 Mar;96(3):795–803. [PubMed] [Google Scholar]
- Mourelle M., Salas A., Guarner F., Crespo E., García-Lafuente A., Malagelada J. R. Stimulation of transforming growth factor beta1 by enteric bacteria in the pathogenesis of rat intestinal fibrosis. Gastroenterology. 1998 Mar;114(3):519–526. doi: 10.1016/s0016-5085(98)70535-9. [DOI] [PubMed] [Google Scholar]
- Riegler M., Lotz M., Sears C., Pothoulakis C., Castagliuolo I., Wang C. C., Sedivy R., Sogukoglu T., Cosentini E., Bischof G. Bacteroides fragilis toxin 2 damages human colonic mucosa in vitro. Gut. 1999 Apr;44(4):504–510. doi: 10.1136/gut.44.4.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitz J., Hecht G., Taveras M., Aoys E., Alverdy J. The effect of dexamethasone administration on rat intestinal permeability: the role of bacterial adherence. Gastroenterology. 1994 Jan;106(1):35–41. doi: 10.1016/s0016-5085(94)94155-6. [DOI] [PubMed] [Google Scholar]
- Spitz J., Yuhan R., Koutsouris A., Blatt C., Alverdy J., Hecht G. Enteropathogenic Escherichia coli adherence to intestinal epithelial monolayers diminishes barrier function. Am J Physiol. 1995 Feb;268(2 Pt 1):G374–G379. doi: 10.1152/ajpgi.1995.268.2.G374. [DOI] [PubMed] [Google Scholar]
- Wells C. L., van de Westerlo E. M., Jechorek R. P., Feltis B. A., Wilkins T. D., Erlandsen S. L. Bacteroides fragilis enterotoxin modulates epithelial permeability and bacterial internalization by HT-29 enterocytes. Gastroenterology. 1996 May;110(5):1429–1437. doi: 10.1053/gast.1996.v110.pm8613048. [DOI] [PubMed] [Google Scholar]
- Wyatt J., Vogelsang H., Hübl W., Waldhöer T., Lochs H. Intestinal permeability and the prediction of relapse in Crohn's disease. Lancet. 1993 Jun 5;341(8858):1437–1439. doi: 10.1016/0140-6736(93)90882-h. [DOI] [PubMed] [Google Scholar]