Abstract
BACKGROUND—Hereditary pancreatitis (HP) is a rare autosomal dominant disorder with variable expression and an overall lifetime penetrance of 80%. We hypothesised that (1) monozygotic twins within similar environments would develop the typical signs of HP at a similar age, and (2) if penetrance were due to modifier genes or environment, all twin pairs would be concordant for expression of HP. AIM—Identify monozygotic twins with HP and determine the penetrance, concordance, and age of onset of symptoms. METHODS—Twins from HP kindreds were identified from the Midwest Multicenter Pancreatic Study group database, referrals, and literature searches. Each twin set was assessed for phenotypic expression, concordance, and difference in age of phenotypic onset of pancreatitis. The difference in onset of symptoms for symptomatic affected non-twin sibling pairs as well as non-twin pairs that were mutation, sex, and age matched were calculated as two comparison groups. RESULTS—Seven of 11 monozygotic pairs identified were suitable for evaluation and four were concordant for pancreatitis. Forty eight affected sibling pairs and 33 pairs of mutation, sex, and age matched (cationic trypsinogen R122H (30 pairs) and N29I (three pairs)) subjects were identified for comparison groups. The median (quartiles Q1, Q3) difference in the age of phenotypic onset in the concordant twins was 1 (0, 2.4) years, 2 (1, 6) for the affected siblings, and 7 (2, 15) years in the comparison control group. Three of the seven sets of twins (43%) were discordant for phenotypic expression of pancreatitis. The overall penetrance in the seven pairs of monozygotic twins was 78.6%. CONCLUSIONS—Genetic and/or environmental factors contribute to expression and age of onset of HP. Nuclear genes or general environmental factors alone cannot explain the 80% penetrance. Determining the mechanism of non-penetrance may help in developing a strategy to prevent the phenotypic expression of pancreatitis in individuals with an underlying genetic predisposition. Keywords: hereditary pancreatitis; genetic; twins; penetrance; trypsinogen
Full Text
The Full Text of this article is available as a PDF (129.8 KB).
Figure 1 .
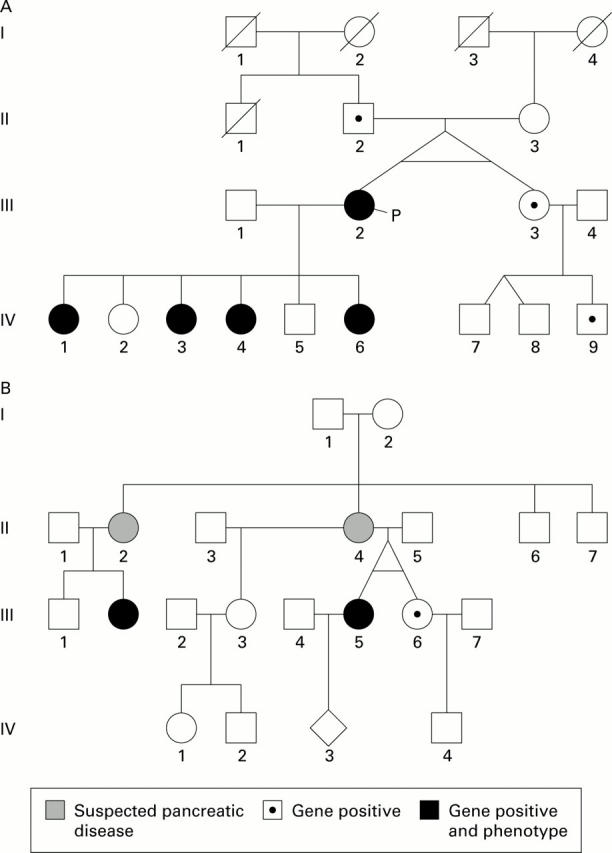
A and B represent kindreds "A" and "G", respectively. In both of these families the twins were raised in the same environment, attended the same college, and lived together until their 20s. Despite identical genes and very similar environments, only one twin of each pair developed phenotypic hereditary pancreatitis.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEALL J. H., Jr, BELL J. W., JESSEPH J. E., NYHUS L. M. Fatal acute hemorrhagic pancreatitis occurring simultaneously in identical twins. Gastroenterology. 1960 Aug;39:215–218. [PubMed] [Google Scholar]
- COMFORT M. W., STEINBERG A. G. Pedigree of a family with hereditary chronic relapsing pancreatitis. Gastroenterology. 1952 May;21(1):54–63. [PubMed] [Google Scholar]
- Dasouki M. J., Cogan J., Summar M. L., Neblitt W., 3rd, Foroud T., Koller D., Phillips J. A., 3rd Heterogeneity in hereditary pancreatitis. Am J Med Genet. 1998 Apr 28;77(1):47–53. [PubMed] [Google Scholar]
- Freud E., Barak R., Ziv N., Leiser A., Dinari G., Mor C., Zer M. Familial chronic recurrent pancreatitis in identical twins. Case report and review of the literature. Arch Surg. 1992 Sep;127(9):1125–1128. doi: 10.1001/archsurg.1992.01420090133020. [DOI] [PubMed] [Google Scholar]
- Gorry M. C., Gabbaizedeh D., Furey W., Gates L. K., Jr, Preston R. A., Aston C. E., Zhang Y., Ulrich C., Ehrlich G. D., Whitcomb D. C. Mutations in the cationic trypsinogen gene are associated with recurrent acute and chronic pancreatitis. Gastroenterology. 1997 Oct;113(4):1063–1068. doi: 10.1053/gast.1997.v113.pm9322498. [DOI] [PubMed] [Google Scholar]
- Gress T. M., Micha A. E., Lacher U., Adler G. Diagnose einer "hereditären Pankreatitis" durch Nachweis der Mutation im kationischen Trypsinogen-Gen. Dtsch Med Wochenschr. 1998 Apr 9;123(15):453–456. doi: 10.1055/s-2007-1023986. [DOI] [PubMed] [Google Scholar]
- Henderson J., Ingram D., House T. Acute pancreatitis in identical twins. Med J Aust. 1982 May 15;1(10):432–434. doi: 10.5694/j.1326-5377.1982.tb132401.x. [DOI] [PubMed] [Google Scholar]
- Kattwinkel J., Lapey A., Di Sant'Agnese P. A., Edwards W. A. Hereditary pancreatitis: three new kindreds and a critical review of the literature. Pediatrics. 1973 Jan;51(1):55–69. [PubMed] [Google Scholar]
- Lalande M. Parental imprinting and human disease. Annu Rev Genet. 1996;30:173–195. doi: 10.1146/annurev.genet.30.1.173. [DOI] [PubMed] [Google Scholar]
- Le Bodic L., Bignon J. D., Raguénès O., Mercier B., Georgelin T., Schnee M., Soulard F., Gagne K., Bonneville F., Muller J. Y. The hereditary pancreatitis gene maps to long arm of chromosome 7. Hum Mol Genet. 1996 Apr;5(4):549–554. doi: 10.1093/hmg/5.4.549. [DOI] [PubMed] [Google Scholar]
- Le Bodic L., Schnee M., Georgelin T., Soulard F., Ferec C., Bignon J. D., Sagniez M. An exceptional genealogy for hereditary chronic pancreatitis. Dig Dis Sci. 1996 Jul;41(7):1504–1510. doi: 10.1007/BF02088580. [DOI] [PubMed] [Google Scholar]
- Mathew P., Wyllie R., Van Lente F., Steffen R. M., Kay M. H. Antioxidants in hereditary pancreatitis. Am J Gastroenterol. 1996 Aug;91(8):1558–1562. [PubMed] [Google Scholar]
- Nishimori I., Kamakura M., Fujikawa-Adachi K., Morita M., Onishi S., Yokoyama K., Makino I., Ishida H., Yamamoto M., Watanabe S. Mutations in exons 2 and 3 of the cationic trypsinogen gene in Japanese families with hereditary pancreatitis. Gut. 1999 Feb;44(2):259–263. doi: 10.1136/gut.44.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandya A., Blanton S. H., Landa B., Javaheri R., Melvin E., Nance W. E., Markello T. Linkage studies in a large kindred with hereditary pancreatitis confirms mapping of the gene to a 16-cM region on 7q. Genomics. 1996 Dec 1;38(2):227–230. doi: 10.1006/geno.1996.0620. [DOI] [PubMed] [Google Scholar]
- Perrault J. Hereditary pancreatitis. Gastroenterol Clin North Am. 1994 Dec;23(4):743–752. [PubMed] [Google Scholar]
- Segal I., Gut A., Schofield D., Shiel N., Braganza J. M. Micronutrient antioxidant status in black South Africans with chronic pancreatitis: opportunity for prophylaxis. Clin Chim Acta. 1995 Jul 31;239(1):71–79. doi: 10.1016/0009-8981(95)06102-j. [DOI] [PubMed] [Google Scholar]
- Sibert J. R. Hereditary pancreatitis in England and Wales. J Med Genet. 1978 Jun;15(3):189–201. doi: 10.1136/jmg.15.3.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sossenheimer M. J., Aston C. E., Preston R. A., Gates L. K., Jr, Ulrich C. D., Martin S. P., Zhang Y., Gorry M. C., Ehrlich G. D., Whitcomb D. C. Clinical characteristics of hereditary pancreatitis in a large family, based on high-risk haplotype. The Midwest Multicenter Pancreatic Study Group (MMPSG) Am J Gastroenterol. 1997 Jul;92(7):1113–1116. [PubMed] [Google Scholar]
- Teich N., Mössner J., Keim V. Mutations of the cationic trypsinogen in hereditary pancreatitis. Hum Mutat. 1998;12(1):39–43. doi: 10.1002/(SICI)1098-1004(1998)12:1<39::AID-HUMU6>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Turker M. S., Bestor T. H. Formation of methylation patterns in the mammalian genome. Mutat Res. 1997 Apr;386(2):119–130. doi: 10.1016/s1383-5742(96)00048-8. [DOI] [PubMed] [Google Scholar]
- Whitcomb D. C. Genetic predispositions to acute and chronic pancreatitis. Med Clin North Am. 2000 May;84(3):531-47, vii. doi: 10.1016/s0025-7125(05)70238-8. [DOI] [PubMed] [Google Scholar]
- Whitcomb D. C., Gorry M. C., Preston R. A., Furey W., Sossenheimer M. J., Ulrich C. D., Martin S. P., Gates L. K., Jr, Amann S. T., Toskes P. P. Hereditary pancreatitis is caused by a mutation in the cationic trypsinogen gene. Nat Genet. 1996 Oct;14(2):141–145. doi: 10.1038/ng1096-141. [DOI] [PubMed] [Google Scholar]
- Whitcomb D. C., Preston R. A., Aston C. E., Sossenheimer M. J., Barua P. S., Zhang Y., Wong-Chong A., White G. J., Wood P. G., Gates L. K., Jr A gene for hereditary pancreatitis maps to chromosome 7q35. Gastroenterology. 1996 Jun;110(6):1975–1980. doi: 10.1053/gast.1996.v110.pm8964426. [DOI] [PubMed] [Google Scholar]
- Witt H., Luck W., Becker M. A signal peptide cleavage site mutation in the cationic trypsinogen gene is strongly associated with chronic pancreatitis. Gastroenterology. 1999 Jul;117(1):7–10. doi: 10.1016/s0016-5085(99)70543-3. [DOI] [PubMed] [Google Scholar]
- Yoder J. A., Bestor T. H. Genetic analysis of genomic methylation patterns in plants and mammals. Biol Chem. 1996 Oct;377(10):605–610. [PubMed] [Google Scholar]


