Abstract
BACKGROUND/AIMS—Hepatocellular carcinoma (HCC) is a common malignant tumour worldwide, and its differential diagnosis from benign lesions of the liver is often difficult yet of great clinical importance. In the present study, we analysed whether glypican-3 is useful in differentiating between benign and malignant liver diseases and whether it influences the growth behaviour of HCC. METHODS—Northern blot analysis and in situ hybridisation. RESULTS—Northern blot analysis indicated that expression of glypican-3 mRNA was either low or absent in normal liver, in focal nodular hyperplasia (FNH), and in liver cirrhosis. In contrast, expression of glypican-3 mRNA was markedly increased in 20 of 30 and moderately increased in five of 30 HCC samples. The average increase in glypican-3 mRNA expression in HCC was significant compared with expression in normal liver (21.7-fold increase, p<0.01). In comparison with FNH or liver cirrhosis, glypican-3 mRNA expression in HCC was increased 7.2- (p<0.05) and 10.8-fold (p<0.01), respectively. In addition, pushing HCCs exhibited significantly higher glypican-3 mRNA expression than invading tumours (p<0.05). In situ hybridisation analysis demonstrated weak expression of glypican-3 mRNA in normal hepatocytes and bile ductular cells, and weak to occasionally moderate signals in hepatocytes forming nodules of liver cirrhosis and in regenerated hepatic nodules of FNH. In contrast, glypican-3 in situ hybridisation signals were intense in hepatic cancer cells with even higher levels in pushing HCCs than in invading HCCs. CONCLUSIONS—These findings suggest that glypican-3, in many cases, has the potential to differentiate between benign and malignant liver diseases. Keywords: focal nodular hyperplasia; liver cirrhosis; hepatocellular cancer; glypican-3
Full Text
The Full Text of this article is available as a PDF (224.0 KB).
Figure 1 .
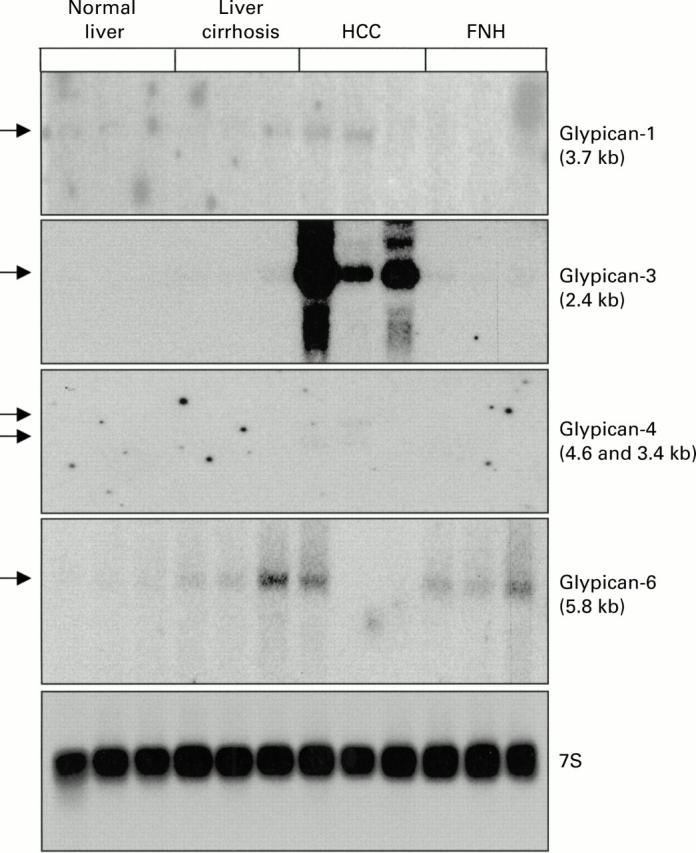
Northern blot analysis of glypican-1, -3, -4, and -6 in normal liver, liver cirrhosis, hepatocellular carcinoma (HCC), and focal nodular hyperplasia (FNH). Total RNA (20 µg) was size fractionated, blotted, and hybridised with the indicated 32P labelled probes.
Figure 2 .
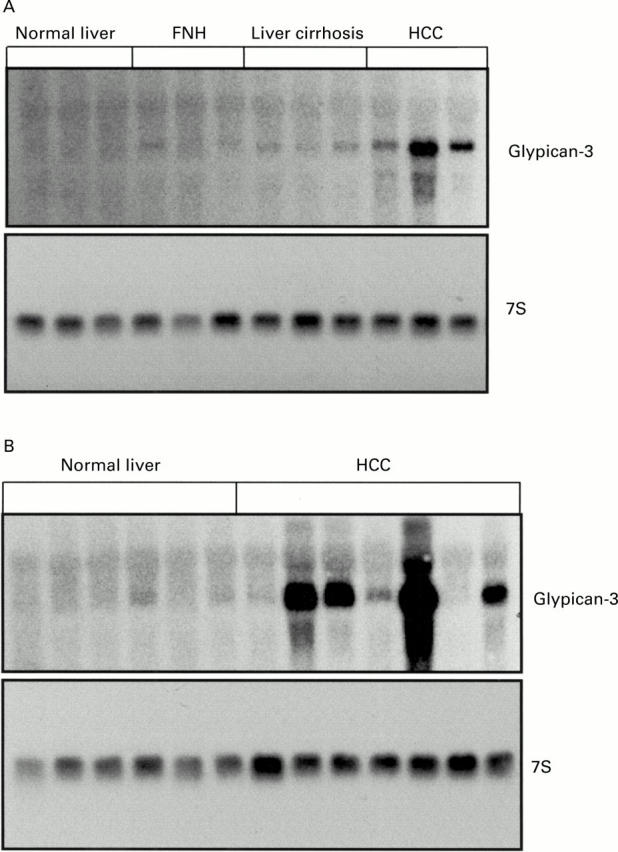
Northern blot analysis. (A) Glypican-3 mRNA in normal liver, focal nodular hyperplasia (FNH), liver cirrhosis, and hepatocellular carcinoma (HCC). (B) Northern blot analysis of glypican-3 mRNA in normal liver and HCC. Total RNA (20 µg) was size fractionated, blotted, and hybridised with 1×106 cpm/ml 32P CTP labelled glypican-3 cRNA probe and 1×105 cpm/ml 32P dCTP 7S cDNA probe.
Figure 3 .
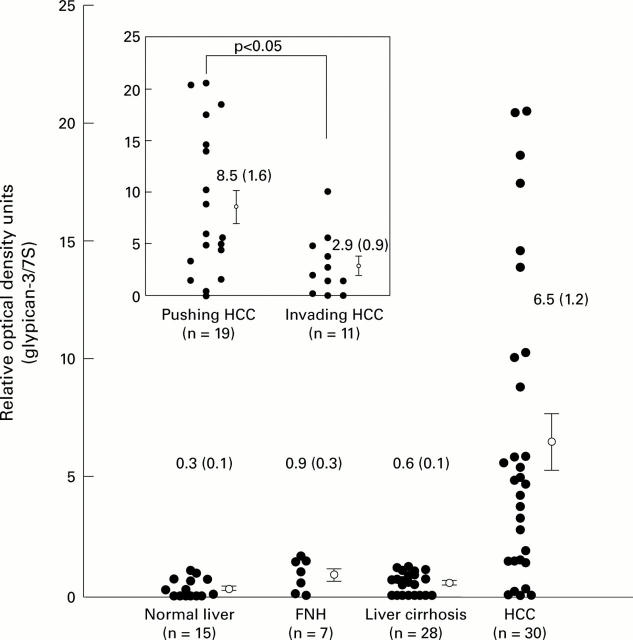
Densitometric analysis. The ratio of the optical density of glypican-3 mRNA to the corresponding 7S signals was calculated and expressed as mean (SEM). FNH, focal nodular hyperplasia; HCC, hepatocellular carcinoma. p<0.01 (HCC v normal); p<0.05 (HCC v FNH); p<0.01 (HCC v liver cirrhosis). Insert: comparison of expression of glypican-3 mRNA between pushing and invading tumours.
Figure 4 .
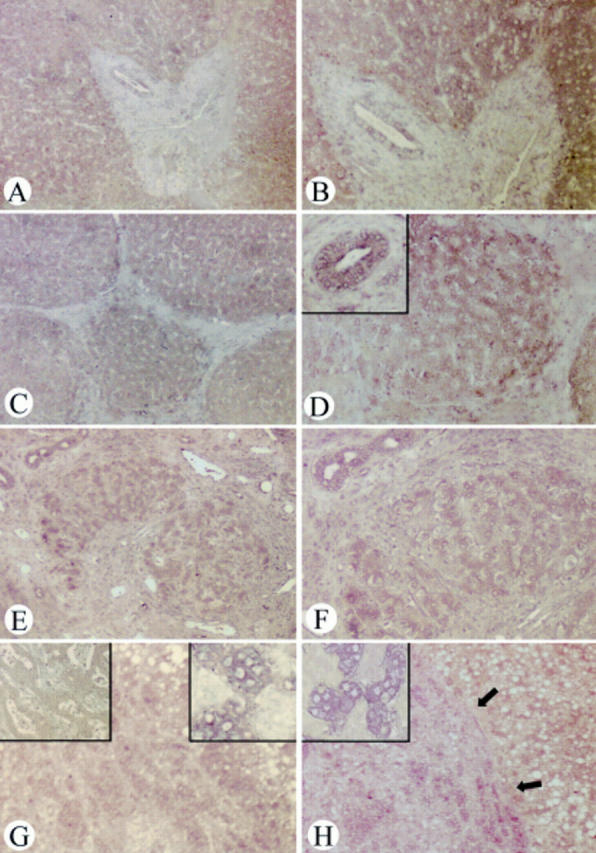
In situ hybridisation of glypican-3 in normal liver tissue (A, B), focal nodular hyperplasia (FNH) (C, D), liver cirrhosis (E, F), and hepatocellular carcinoma (HCC) (G, H). In normal liver tissues, weak glypican-3 mRNA signals were present in bile ductal cells and in hepatocytes (magnification: A ×50, B ×100). In FNH (magnification: C ×50, D ×100; D insert, bile duct adjacent to FNH tissue ×100) and liver cirrhosis (magnification: E ×50, F ×100), glypican-3 mRNA signals were weakly to occasionally moderately present in hepatocytes and weakly to moderately present in bile ductal cells in liver cirrhosis and in bile ductal cells adjacent to FNH tissue. In HCC, glypican-3 mRNA was more intensely present in tumours with a pushing growth behaviour (magnification: H ×50, H insert ×200) than in those with an invading growth behaviour (magnification: G ×50, G upper left insert ×200). In situ hybridisation in HCC with the glypican-3 sense probe showed no signal (magnification: G insert ×100).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnoletti J. P., Brodsky J. Surgical treatment of benign hepatic mass lesions. Am Surg. 1999 May;65(5):431–433. [PubMed] [Google Scholar]
- Baer H. U., Gertsch P., Matthews J. B., Schweizer W., Triller J., Zimmermann A., Blumgart L. H. Resectability of large focal liver lesions. Br J Surg. 1989 Oct;76(10):1042–1044. doi: 10.1002/bjs.1800761019. [DOI] [PubMed] [Google Scholar]
- Bassel K., Lee M., Seymour N. E. Focal nodular hyperplasia of the liver. South Med J. 1994 Sep;87(9):918–920. doi: 10.1097/00007611-199409000-00011. [DOI] [PubMed] [Google Scholar]
- Ferrell L. Hepatocellular nodules in the cirrhotic liver: diagnostic features and proposed nomenclature. Pathology (Phila) 1994;3(1):105–117. [PubMed] [Google Scholar]
- Friess H., Lu Z., Graber H. U., Zimmermann A., Adler G., Korc M., Schmid R. M., Büchler M. W. bax, but not bcl-2, influences the prognosis of human pancreatic cancer. Gut. 1998 Sep;43(3):414–421. doi: 10.1136/gut.43.3.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez A. D., Kaya M., Shi W., Song H., Testa J. R., Penn L. Z., Filmus J. OCI-5/GPC3, a glypican encoded by a gene that is mutated in the Simpson-Golabi-Behmel overgrowth syndrome, induces apoptosis in a cell line-specific manner. J Cell Biol. 1998 Jun 15;141(6):1407–1414. doi: 10.1083/jcb.141.6.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamazaki K., Yunoki Y., Tagashira H., Mimura T., Mori M., Orita K. Epidermal growth factor receptor in human hepatocellular carcinoma. Cancer Detect Prev. 1997;21(4):355–360. [PubMed] [Google Scholar]
- Haydon G. H., Hayes P. C. Screening for hepatocellular carcinoma. Eur J Gastroenterol Hepatol. 1996 Sep;8(9):856–860. [PubMed] [Google Scholar]
- Hisaka T., Yano H., Haramaki M., Utsunomiya I., Kojiro M. Expressions of epidermal growth factor family and its receptor in hepatocellular carcinoma cell lines: relationship to cell proliferation. Int J Oncol. 1999 Mar;14(3):453–460. doi: 10.3892/ijo.14.3.453. [DOI] [PubMed] [Google Scholar]
- Hsu H. C., Cheng W., Lai P. L. Cloning and expression of a developmentally regulated transcript MXR7 in hepatocellular carcinoma: biological significance and temporospatial distribution. Cancer Res. 1997 Nov 15;57(22):5179–5184. [PubMed] [Google Scholar]
- Hytiroglou P., Theise N. D. Differential diagnosis of hepatocellular nodular lesions. Semin Diagn Pathol. 1998 Nov;15(4):285–299. [PubMed] [Google Scholar]
- Kleeff J., Ishiwata T., Kumbasar A., Friess H., Büchler M. W., Lander A. D., Korc M. The cell-surface heparan sulfate proteoglycan glypican-1 regulates growth factor action in pancreatic carcinoma cells and is overexpressed in human pancreatic cancer. J Clin Invest. 1998 Nov 1;102(9):1662–1673. doi: 10.1172/JCI4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H., Huber R., Schlessinger D., Morin P. J. Frequent silencing of the GPC3 gene in ovarian cancer cell lines. Cancer Res. 1999 Feb 15;59(4):807–810. [PubMed] [Google Scholar]
- Murthy S. S., Shen T., De Rienzo A., Lee W. C., Ferriola P. C., Jhanwar S. C., Mossman B. T., Filmus J., Testa J. R. Expression of GPC3, an X-linked recessive overgrowth gene, is silenced in malignant mesothelioma. Oncogene. 2000 Jan 20;19(3):410–416. doi: 10.1038/sj.onc.1203322. [DOI] [PubMed] [Google Scholar]
- Paine-Saunders S., Viviano B. L., Saunders S. GPC6, a novel member of the glypican gene family, encodes a product structurally related to GPC4 and is colocalized with GPC5 on human chromosome 13. Genomics. 1999 May 1;57(3):455–458. doi: 10.1006/geno.1999.5793. [DOI] [PubMed] [Google Scholar]
- Pellegrini M., Pilia G., Pantano S., Lucchini F., Uda M., Fumi M., Cao A., Schlessinger D., Forabosco A. Gpc3 expression correlates with the phenotype of the Simpson-Golabi-Behmel syndrome. Dev Dyn. 1998 Dec;213(4):431–439. doi: 10.1002/(SICI)1097-0177(199812)213:4<431::AID-AJA8>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Pergolizzi J. V., Jr, Auster M., Conaway G. L., Sardi A. Cryosurgery for unresectable primary hepatocellular carcinoma: a case report and review of literature. Am Surg. 1999 May;65(5):402–405. [PubMed] [Google Scholar]
- Pilia G., Hughes-Benzie R. M., MacKenzie A., Baybayan P., Chen E. Y., Huber R., Neri G., Cao A., Forabosco A., Schlessinger D. Mutations in GPC3, a glypican gene, cause the Simpson-Golabi-Behmel overgrowth syndrome. Nat Genet. 1996 Mar;12(3):241–247. doi: 10.1038/ng0396-241. [DOI] [PubMed] [Google Scholar]
- Roskams T., De Vos R., David G., Van Damme B., Desmet V. Heparan sulphate proteoglycan expression in human primary liver tumours. J Pathol. 1998 Jul;185(3):290–297. doi: 10.1002/(SICI)1096-9896(199807)185:3<290::AID-PATH91>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Roskams T., Moshage H., De Vos R., Guido D., Yap P., Desmet V. Heparan sulfate proteoglycan expression in normal human liver. Hepatology. 1995 Apr;21(4):950–958. [PubMed] [Google Scholar]
- Roskams T., Rosenbaum J., De Vos R., David G., Desmet V. Heparan sulfate proteoglycan expression in chronic cholestatic human liver diseases. Hepatology. 1996 Sep;24(3):524–532. doi: 10.1053/jhep.1996.v24.pm0008781318. [DOI] [PubMed] [Google Scholar]
- Ueki T., Fujimoto J., Suzuki T., Yamamoto H., Okamoto E. Expression of hepatocyte growth factor and its receptor c-met proto-oncogene in hepatocellular carcinoma. Hepatology. 1997 Apr;25(4):862–866. doi: 10.1002/hep.510250413. [DOI] [PubMed] [Google Scholar]