Abstract
BACKGROUND AND AIMS—It has been suggested that the analgesic effect of the somatostatin analogue octreotide in visceral pain involves peripheral mechanisms. We evaluated the effect of octreotide on responses to noxious colorectal distension in rats. METHODS—In a behavioural study, pressor and electromyographic responses to colorectal distension were evaluated before and after intravenous or intrathecal administration of octreotide. In pelvic nerve afferent fibre recordings, responses of mechanosensitive fibres innervating the colon to noxious colorectal distension (80 mm Hg, 30 seconds) were tested before and after octreotide. RESULTS—Octreotide was ineffective in attenuating responses to colorectal distension in either normal or acetic acid inflamed colon when administered intravenously but attenuated responses when given intrathecally. Administration of octreotide over a broad dose range (0.5 µg/kg to 2.4 mg/kg) did not alter responses of afferent fibres to noxious colorectal distension in untreated, or acetic acid or zymosan treated colons. CONCLUSIONS—In the rat, octreotide has no peripheral (pelvic nerve) modulatory action in visceral nociception. The antinociceptive effect of octreotide in this model of visceral nociception is mediated by an action at central sites. Keywords: octreotide; colorectal distension; electromyographic responses; afferent fibres; visceral pain; analgesic effect; rat
Full Text
The Full Text of this article is available as a PDF (173.1 KB).
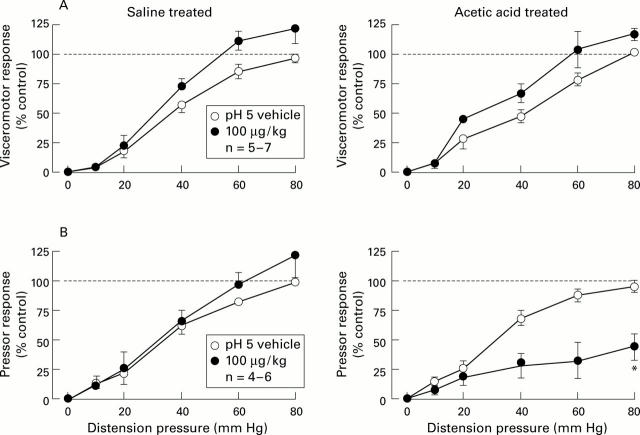
Figure 1 .
Stimulus-response functions (SRFs) for visceromotor (A) and pressor (B) responses to colorectal distension before (vehicle) and after intravenous administration of octreotide (100 µg) in saline and acetic acid treated rats. The broken line represents the control response to 80 mm Hg distension as 100%. All data are expressed as percentage of maximal control response (mean (SEM)). The basis for n is the number of rats used in constructing each SRF. *Significantly different from control group (ANOVA).
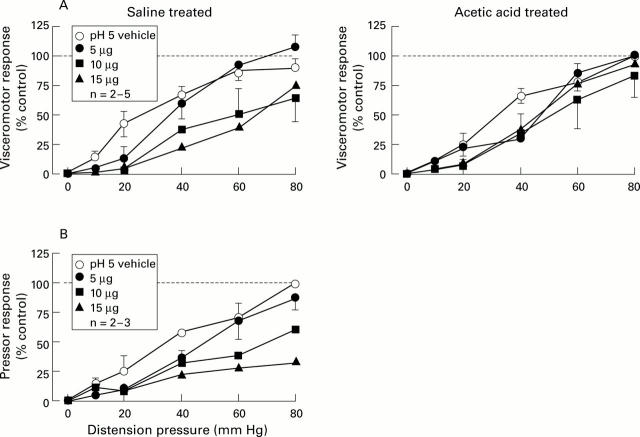
Figure 2 .
Stimulus response functions (SRFs) for visceromotor (A) and pressor (B) responses to colorectal distension (CRD) before (vehicle) and after cumulative doses of intrathecal administration of octreotide (5-15 µg) in saline and acetic acid treated rats. The broken line represents the control response to 80 mm Hg distension as 100%. All data are expressed as percentage of control response (mean (SEM)). The basis for n is the number of rats used in constructing each SRF. Intrathecal octreotide dose dependently attenuated the visceromotor (A) and pressor (B) responses to CRD in saline treated rats.
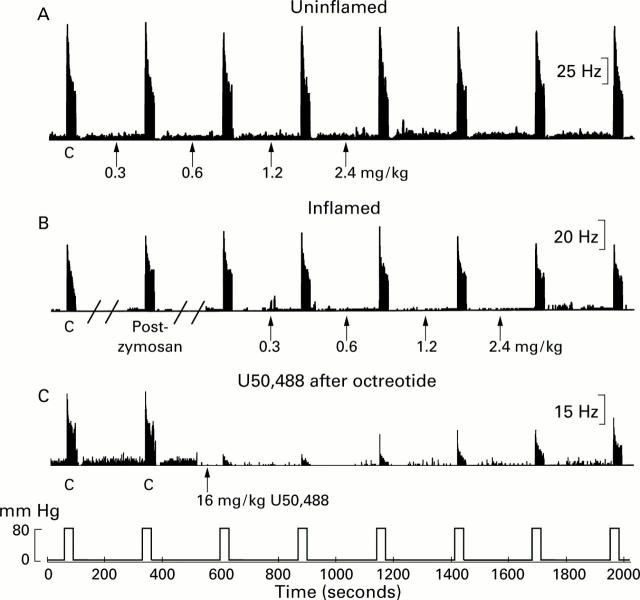
Figure 3 .
Effects of cumulative systemic administration of octreotide on responses of pelvic nerve afferent fibres to noxious colorectal distension (CRD) (80 mm Hg, 30 seconds). Examples of lack of effect of octreotide on responses of different Aδ afferent fibres to CRD in uninflamed colon (A) and zymosan inflamed colon (B). Thirty minutes after intracolonic injection of zymosan (25 mg/ml, 2 ml in 30% ethanol), the fibre in (B) was sensitised (response magnitude increased from 679 imp/30s to 897 imp/30s, an increase to 132% of the pre-zymosan control ("C") response). Octreotide was injected intra-arterially in a cumulative dose as indicated by the arrows. Responses of the fibres are illustrated as peristimulus time histograms (one second binwidth); distending pressure (80 mm Hg, 30 seconds every four minutes) is presented below. In (C), the effect of U50,488 (16 mg/kg) is shown for a fibre that had previously received cumulative doses of octreotide (2.4 mg/kg).
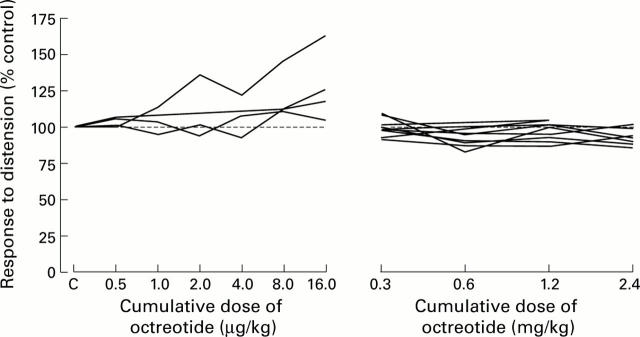
Figure 4 .
Absence of dose dependent effects of octreotide on responses of individual pelvic nerve afferent fibres to noxious colorectal distension (80 mm Hg, 30 seconds, as percentage of control). Octreotide failed to attenuate responses of pelvic nerve afferent fibres recorded from either untreated or zymosan treated colon.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Betoin F., Ardid D., Herbet A., Aumaitre O., Kemeny J. L., Duchene-Marullaz P., Lavarenne J., Eschalier A. Evidence for a central long-lasting antinociceptive effect of vapreotide, an analog of somatostatin, involving an opioidergic mechanism. J Pharmacol Exp Ther. 1994 Apr;269(1):7–14. [PubMed] [Google Scholar]
- Bradette M., Delvaux M., Staumont G., Fioramonti J., Bueno L., Frexinos J. Octreotide increases thresholds of colonic visceral perception in IBS patients without modifying muscle tone. Dig Dis Sci. 1994 Jun;39(6):1171–1178. doi: 10.1007/BF02093780. [DOI] [PubMed] [Google Scholar]
- Bruno J. F., Xu Y., Song J., Berelowitz M. Tissue distribution of somatostatin receptor subtype messenger ribonucleic acid in the rat. Endocrinology. 1993 Dec;133(6):2561–2567. doi: 10.1210/endo.133.6.8243278. [DOI] [PubMed] [Google Scholar]
- Burton M. B., Gebhart G. F. Effects of intracolonic acetic acid on responses to colorectal distension in the rat. Brain Res. 1995 Feb 20;672(1-2):77–82. doi: 10.1016/0006-8993(94)01382-r. [DOI] [PubMed] [Google Scholar]
- Burton M. B., Gebhart G. F. Effects of kappa-opioid receptor agonists on responses to colorectal distension in rats with and without acute colonic inflammation. J Pharmacol Exp Ther. 1998 May;285(2):707–715. [PubMed] [Google Scholar]
- Chapman V., Dickenson A. H. The effects of sandostatin and somatostatin on nociceptive transmission in the dorsal horn of the rat spinal cord. Neuropeptides. 1992 Nov;23(3):147–152. doi: 10.1016/0143-4179(92)90115-d. [DOI] [PubMed] [Google Scholar]
- Chey W. D., Beydoun A., Roberts D. J., Hasler W. L., Owyang C. Octreotide reduces perception of rectal electrical stimulation by spinal afferent pathway inhibition. Am J Physiol. 1995 Dec;269(6 Pt 1):G821–G826. doi: 10.1152/ajpgi.1995.269.6.G821. [DOI] [PubMed] [Google Scholar]
- Chrubasik J., Meynadier J., Blond S., Scherpereel P., Ackerman E., Weinstock M., Bonath K., Cramer H., Wünsch E. Somatostatin, a potent analgesic. Lancet. 1984 Nov 24;2(8413):1208–1209. doi: 10.1016/s0140-6736(84)92761-2. [DOI] [PubMed] [Google Scholar]
- Coutinho S. V., Su X., Sengupta J. N., Gebhart G. F. Role of sensitized pelvic nerve afferents from the inflamed rat colon in the maintenance of visceral hyperalgesia. Prog Brain Res. 2000;129:375–387. doi: 10.1016/S0079-6123(00)29029-8. [DOI] [PubMed] [Google Scholar]
- Davies A. M., Lumsden A. G. Fasciculation in the early mouse trigeminal nerve is not ordered in relation to the emerging pattern of whisker follicles. J Comp Neurol. 1986 Nov 1;253(1):13–24. doi: 10.1002/cne.902530103. [DOI] [PubMed] [Google Scholar]
- Gaumann D. M., Yaksh T. L., Post C., Wilcox G. L., Rodriguez M. Intrathecal somatostatin in cat and mouse studies on pain, motor behavior, and histopathology. Anesth Analg. 1989 May;68(5):623–632. [PubMed] [Google Scholar]
- Green T., Dockray G. J. Characterization of the peptidergic afferent innervation of the stomach in the rat, mouse and guinea-pig. Neuroscience. 1988 Apr;25(1):181–193. doi: 10.1016/0306-4522(88)90017-6. [DOI] [PubMed] [Google Scholar]
- Hanesch U., Heppelmann B., Schmidt R. F. Somatostatin-like immunoreactivity in primary afferents of the medial articular nerve and colocalization with substance P in the cat. J Comp Neurol. 1995 Apr 10;354(3):345–352. doi: 10.1002/cne.903540304. [DOI] [PubMed] [Google Scholar]
- Hasler W. L., Soudah H. C., Owyang C. A somatostatin analogue inhibits afferent pathways mediating perception of rectal distention. Gastroenterology. 1993 May;104(5):1390–1397. doi: 10.1016/0016-5085(93)90347-f. [DOI] [PubMed] [Google Scholar]
- Hasler W. L., Soudah H. C., Owyang C. Somatostatin analog inhibits afferent response to rectal distention in diarrhea-predominant irritable bowel patients. J Pharmacol Exp Ther. 1994 Mar;268(3):1206–1211. [PubMed] [Google Scholar]
- Heppelmann B., Pawlak M. Inhibitory effect of somatostatin on the mechanosensitivity of articular afferents in normal and inflamed knee joints of the rat. Pain. 1997 Dec;73(3):377–382. doi: 10.1016/S0304-3959(97)00124-3. [DOI] [PubMed] [Google Scholar]
- Hicks G. A., Feniuk W., Humphrey P. P. Outward current produced by somatostatin (SRIF) in rat anterior cingulate pyramidal cells in vitro. Br J Pharmacol. 1998 May;124(1):252–258. doi: 10.1038/sj.bjp.0701824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hökfelt T., Elde R., Johansson O., Luft R., Nilsson G., Arimura A. Immunohistochemical evidence for separate populations of somatostatin-containing and substance P-containing primary afferent neurons in the rat. Neuroscience. 1976;1(2):131–136. doi: 10.1016/0306-4522(76)90008-7. [DOI] [PubMed] [Google Scholar]
- Inagaki S., Kito S. Peptides in the peripheral nervous system. Prog Brain Res. 1986;66:269–316. doi: 10.1016/s0079-6123(08)64607-5. [DOI] [PubMed] [Google Scholar]
- Johnston B. T., Shils J., Leite L. P., Castell D. O. Effects of octreotide on esophageal visceral perception and cerebral evoked potentials induced by balloon distension. Am J Gastroenterol. 1999 Jan;94(1):65–70. doi: 10.1111/j.1572-0241.1999.00772.x. [DOI] [PubMed] [Google Scholar]
- Leblanc R., Gauthier S., Gauvin M., Quirion R., Palmour R., Masson H. Neurobehavioral effects of intrathecal somatostatinergic treatment in subhuman primates. Neurology. 1988 Dec;38(12):1887–1890. doi: 10.1212/wnl.38.12.1887. [DOI] [PubMed] [Google Scholar]
- Mayer E. A., Gebhart G. F. Basic and clinical aspects of visceral hyperalgesia. Gastroenterology. 1994 Jul;107(1):271–293. doi: 10.1016/0016-5085(94)90086-8. [DOI] [PubMed] [Google Scholar]
- Molander C., Ygge J., Dalsgaard C. J. Substance P-, somatostatin- and calcitonin gene-related peptide-like immunoreactivity and fluoride resistant acid phosphatase-activity in relation to retrogradely labeled cutaneous, muscular and visceral primary sensory neurons in the rat. Neurosci Lett. 1987 Feb 10;74(1):37–42. doi: 10.1016/0304-3940(87)90047-4. [DOI] [PubMed] [Google Scholar]
- Mollenholt P., Post C., Rawal N., Freedman J., Hökfelt T., Paulsson I. Antinociceptive and 'neurotoxic' actions of somatostatin in rat spinal cord after intrathecal administration. Pain. 1988 Jan;32(1):95–105. doi: 10.1016/0304-3959(88)90028-0. [DOI] [PubMed] [Google Scholar]
- Morton C. R., Hutchison W. D., Hendry I. A., Duggan A. W. Somatostatin: evidence for a role in thermal nociception. Brain Res. 1989 May 29;488(1-2):89–96. doi: 10.1016/0006-8993(89)90696-3. [DOI] [PubMed] [Google Scholar]
- Murase K., Nedeljkov V., Randić M. The actions of neuropeptides on dorsal horn neurons in the rat spinal cord slice preparation: an intracellular study. Brain Res. 1982 Feb 18;234(1):170–176. doi: 10.1016/0006-8993(82)90483-8. [DOI] [PubMed] [Google Scholar]
- Nakabayashi H., Kobayashi K., Nakabayashi I. O., Kurata Y. Somatostatin receptor on the afferent nerve terminals in the rat hepatoportal area. Neurosci Lett. 1995 Jan 2;183(1-2):46–49. doi: 10.1016/0304-3940(94)11111-u. [DOI] [PubMed] [Google Scholar]
- Ness T. J., Gebhart G. F. Colorectal distension as a noxious visceral stimulus: physiologic and pharmacologic characterization of pseudaffective reflexes in the rat. Brain Res. 1988 May 31;450(1-2):153–169. doi: 10.1016/0006-8993(88)91555-7. [DOI] [PubMed] [Google Scholar]
- Paice J. A., Penn R. D., Kroin J. S. Intrathecal octreotide for relief of intractable nonmalignant pain: 5-year experience with two cases. Neurosurgery. 1996 Jan;38(1):203–207. doi: 10.1097/00006123-199601000-00047. [DOI] [PubMed] [Google Scholar]
- Penn R. D., Paice J. A., Kroin J. S. Octreotide: a potent new non-opiate analgesic for intrathecal infusion. Pain. 1992 Apr;49(1):13–19. doi: 10.1016/0304-3959(92)90182-B. [DOI] [PubMed] [Google Scholar]
- Plourde V., Lembo T., Shui Z., Parker J., Mertz H., Taché Y., Sytnik B., Mayer E. Effects of the somatostatin analogue octreotide on rectal afferent nerves in humans. Am J Physiol. 1993 Oct;265(4 Pt 1):G742–G751. doi: 10.1152/ajpgi.1993.265.4.G742. [DOI] [PubMed] [Google Scholar]
- Proudlock F., Spike R. C., Todd A. J. Immunocytochemical study of somatostatin, neurotensin, GABA, and glycine in rat spinal dorsal horn. J Comp Neurol. 1993 Jan 8;327(2):289–297. doi: 10.1002/cne.903270210. [DOI] [PubMed] [Google Scholar]
- Radulovic S. S., Milovanovic S. R., Cai R. Z., Schally A. V. The binding of bombesin and somatostatin and their analogs to human colon cancers. Proc Soc Exp Biol Med. 1992 Jul;200(3):394–401. doi: 10.3181/00379727-200-43447. [DOI] [PubMed] [Google Scholar]
- Randić M., Miletić V. Depressant actions of methionine-enkephalin and somatostatin in cat dorsal horn neurones activated by noxious stimuli. Brain Res. 1978 Aug 18;152(1):196–202. doi: 10.1016/0006-8993(78)90148-8. [DOI] [PubMed] [Google Scholar]
- Reubi J. C., Horisberger U., Waser B., Gebbers J. O., Laissue J. Preferential location of somatostatin receptors in germinal centers of human gut lymphoid tissue. Gastroenterology. 1992 Oct;103(4):1207–1214. doi: 10.1016/0016-5085(92)91505-x. [DOI] [PubMed] [Google Scholar]
- Reubi J. C., Laissue J., Waser B., Horisberger U., Schaer J. C. Expression of somatostatin receptors in normal, inflamed, and neoplastic human gastrointestinal tissues. Ann N Y Acad Sci. 1994 Sep 15;733:122–137. doi: 10.1111/j.1749-6632.1994.tb17262.x. [DOI] [PubMed] [Google Scholar]
- Reubi J. C., Mazzucchelli L., Laissue J. A. Intestinal vessels express a high density of somatostatin receptors in human inflammatory bowel disease. Gastroenterology. 1994 Apr;106(4):951–959. doi: 10.1016/0016-5085(94)90754-4. [DOI] [PubMed] [Google Scholar]
- Ribeiro-da-Silva A., Cuello A. C. Ultrastructural evidence for the occurrence of two distinct somatostatin-containing systems in the substantia gelatinosa of rat spinal cord. J Chem Neuroanat. 1990 Mar-Apr;3(2):141–153. [PubMed] [Google Scholar]
- Sandkühler J., Fu Q. G., Helmchen C. Spinal somatostatin superfusion in vivo affects activity of cat nociceptive dorsal horn neurons: comparison with spinal morphine. Neuroscience. 1990;34(3):565–576. doi: 10.1016/0306-4522(90)90165-z. [DOI] [PubMed] [Google Scholar]
- Señaris R. M., Schindler M., Humphrey P. P., Emson P. C. Expression of somatostatin receptor 3 mRNA in the motorneurones of the rat spinal cord, and the sensory neurones of the spinal ganglia. Brain Res Mol Brain Res. 1995 Mar;29(1):185–190. doi: 10.1016/0169-328x(94)00275-j. [DOI] [PubMed] [Google Scholar]
- Su X., Sengupta J. N., Gebhart G. F. Effects of kappa opioid receptor-selective agonists on responses of pelvic nerve afferents to noxious colorectal distension. J Neurophysiol. 1997 Aug;78(2):1003–1012. doi: 10.1152/jn.1997.78.2.1003. [DOI] [PubMed] [Google Scholar]
- Su X., Sengupta J. N., Gebhart G. F. Effects of opioids on mechanosensitive pelvic nerve afferent fibers innervating the urinary bladder of the rat. J Neurophysiol. 1997 Mar;77(3):1566–1580. doi: 10.1152/jn.1997.77.3.1566. [DOI] [PubMed] [Google Scholar]
- Traub R. J., Hutchcroft K., Gebhart G. F. The peptide content of colonic afferents decreases following colonic inflammation. Peptides. 1999;20(2):267–273. doi: 10.1016/s0196-9781(98)00157-0. [DOI] [PubMed] [Google Scholar]
- Tuchscherer M. M., Seybold V. S. A quantitative study of the coexistence of peptides in varicosities within the superficial laminae of the dorsal horn of the rat spinal cord. J Neurosci. 1989 Jan;9(1):195–205. doi: 10.1523/JNEUROSCI.09-01-00195.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]