Abstract
BACKGROUND—TFF2, a member of the trefoil factor family of proteins, is a glycosylated protein of 106 amino acids. It is secreted by gastric antral and pyloric glands and by Brunner's glands of the duodenum. TFF2 is found in high concentrations around sites of ulceration. It stimulates cell motility and is probably the principal cytoprotective trefoil peptide in the stomach. AIMS—To determine if production of TFF2 follows a circadian rhythm and to measure changes in secretion of TFF2 in response to food intake and during sleep. SUBJECTS—Young healthy adults were recruited. They were asymptomatic and were not receiving medication. The 24 hour regimen was designed to allow normal stimulation of gastric secretion in response to food intake and sleep. Gastric juice was collected two hourly via a nasogastric tube. METHODS—Glycosylated and non-glycosylated TFF2 proteins were measured by quantitative western transfer analysis. The results were analysed statistically using SPSS software. RESULTS—There was a dramatic diurnal variation in the concentration of TFF2. The mean concentration was lowest in the early evening (0.29 µg/ml), increased gradually during the evening, and then sharply during the night to reach 7.9 µg/ml. The ratio of glycosylated to non-glycosylated TFF2 varied and was higher during the night than in the afternoon. pH, total protein, and pepsin concentrations in gastric juice did not vary significantly over 24 hours. CONCLUSION—The data suggest that diurnal variations in TFF2 secretion occur independently of pepsin and gastric acid secretion. The concentration of glycosylated TFF2 in the gastric lumen falls in response to food intake. TFF2 secretion increases during inactivity and sleep. These results suggest that secretion of TFF2 in the stomach is highest during the night and that the cytoprotective effects of TFF2 on the gastric mucosa occur mainly during sleep. Keywords: trefoil protein; human TFF2; gastric acid; diurnal; pepsin; circadian rhythm
Full Text
The Full Text of this article is available as a PDF (190.0 KB).
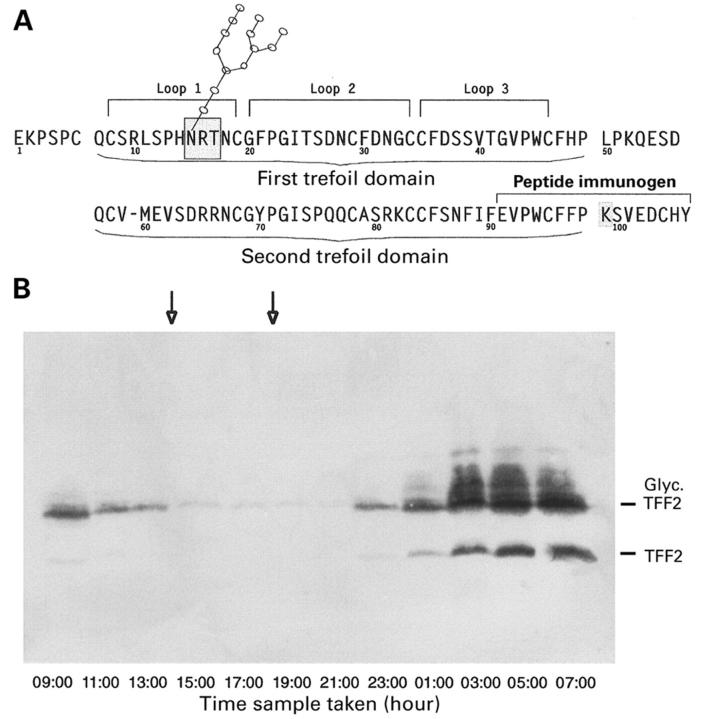
Figure 1 .
Detection of TFF2 protein in gastric juice over 24 hours. (A) Modified sequence of the mature TFF2 protein. The positions of the two trefoil domains are indicated, as are the amino acids included in each of the three loops formed during folding of the trefoil domains. The single N-glycosylation recognition sequence, which is a potential glycosylation site located in loop 1 of the first trefoil domain, is boxed. The lysine residue (K) which replaces the asparagine residue found in the previously published sequence1 at position 99 is also boxed. The 16 amino acid peptide synthesised for use as an immunogen is also indicated. Spaces have been inserted in the sequence after residues 6, 49, and 98 to indicate the limits of the trefoil domains. (B) Human gastric juice was collected at two hourly intervals over a 24 hour time course from a young volunteer. Aliquots were subjected to electrophoresis on a denaturing polyacrylamide gel, transferred to PVDF membranes, and reacted with TFF2 antisera as described in the methods section. Downward pointing arrows indicate meal times. The positions of glycosylated TFF2 (Glyc. TFF2) and non-glycosylated TFF2 (TFF2) are shown on the right hand side.
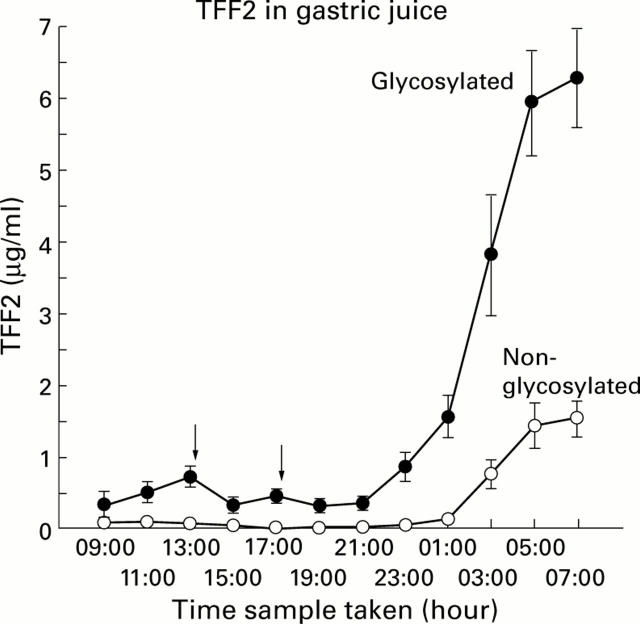
Figure 2 .
Concentration of glycosylated TFF2 and non-glycosylated TFF2 in normal human gastric juice over a 24 hour period. Gastric juice was collected from 12 healthy volunteers at two hourly intervals for 24 hours and concentrations of TFF2 were measured as described in the methods. Mean (SEM) concentrations of glycosylated TFF2 and non-glycosylated TFF2 are shown. Downward pointing arrows indicate meal times.
Figure 3 .
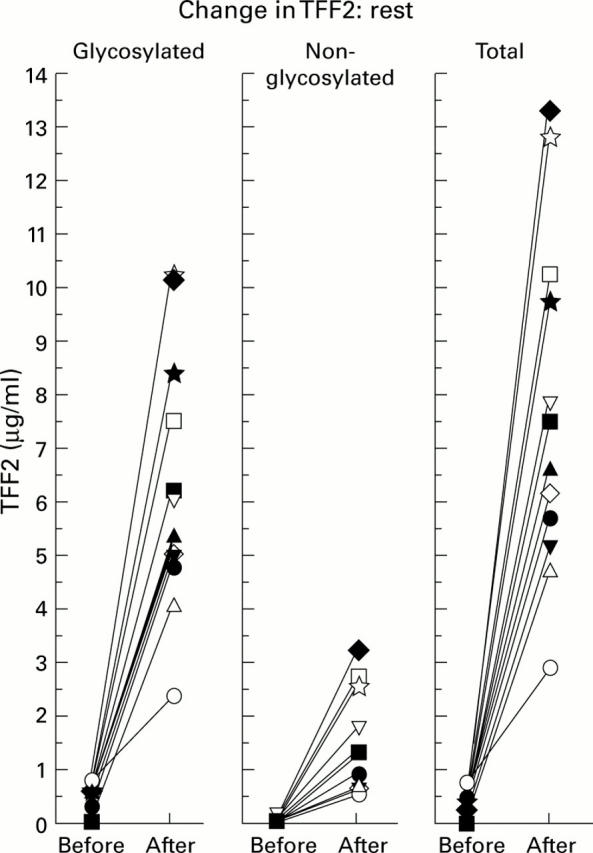
Effect of sleep on gastric luminal TFF2 concentrations. Gastric juice was collected from 12 healthy volunteers in the early evening at 7:00 pm and after they woke at 7:00 am. Concentrations of glycosylated TFF2, non-glycosylated TFF2, and total TFF2 concentrations were measured as described in the methods. The values obtained for all volunteers are shown.
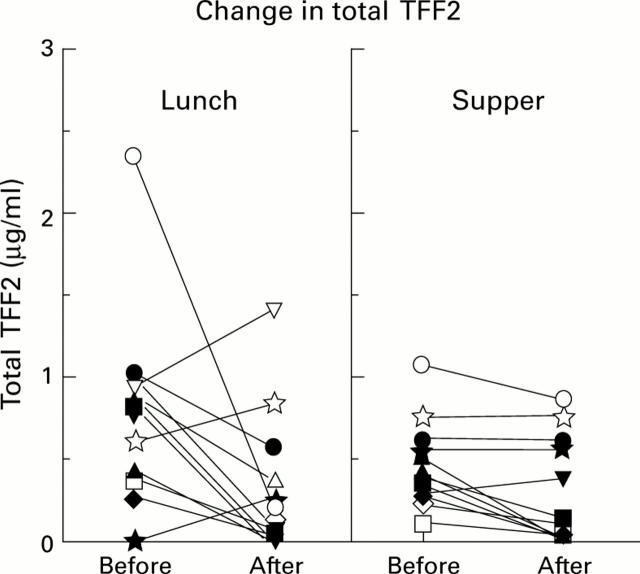
Figure 4 .
Effect of eating on gastric luminal TFF2 concentrations. Gastric juice was collected from 12 healthy volunteers 15 minutes before and one hour and 45 minutes after both lunch and supper. Total TFF2 concentrations were measured as described in the methods. Values obtained for all volunteers are shown.
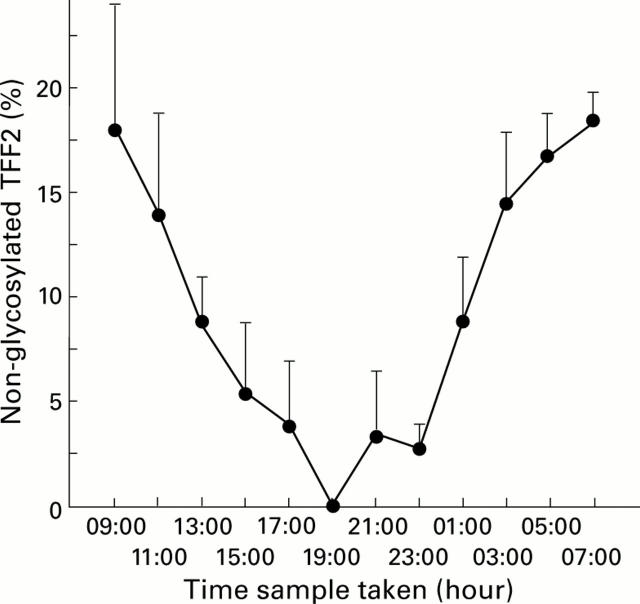
Figure 5 .
Proportion of TFF2 that is not glycosylated. Gastric juice was collected from 12 healthy volunteers at two hourly intervals for 24 hours and concentrations of TFF2 were measured as described in the methods. Mean (SEM) percentages of the total TFF2 that was not glycosylated are shown.
Figure 6 .
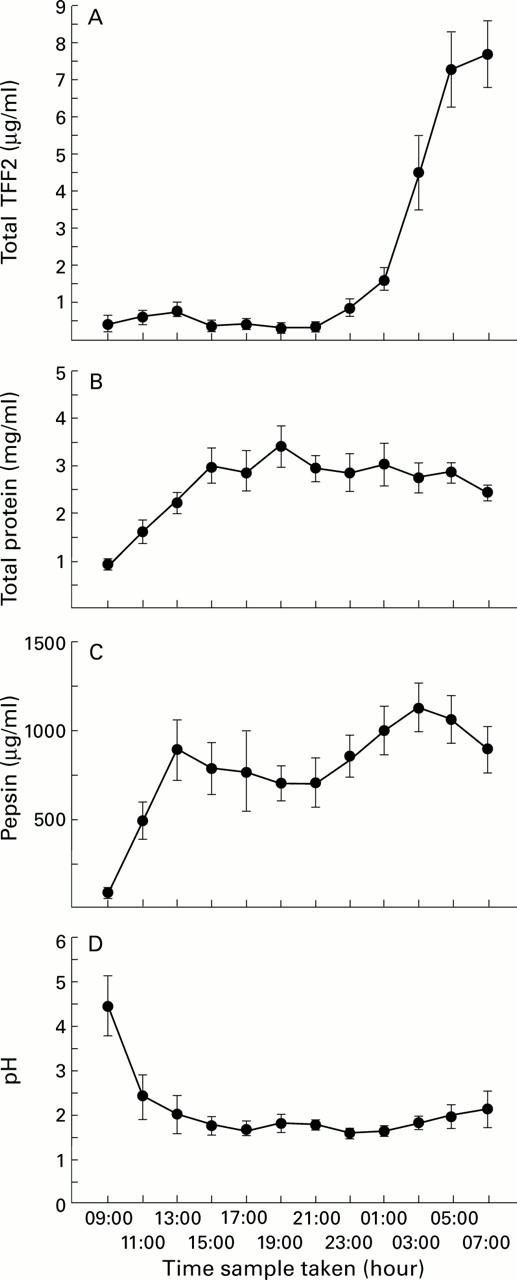
Comparison of total TFF2 concentration with total protein concentration, pepsin activity, and pH in normal human gastric juice over 24 hours. Gastric juice was collected from 12 young volunteers at two hourly intervals for 24 hours and concentrations of TFF2 (A) and protein (B), pepsin activity (C), and pH (D) of the samples were measured as described in the methods. Mean (SEM) values are shown.
Figure 7 .
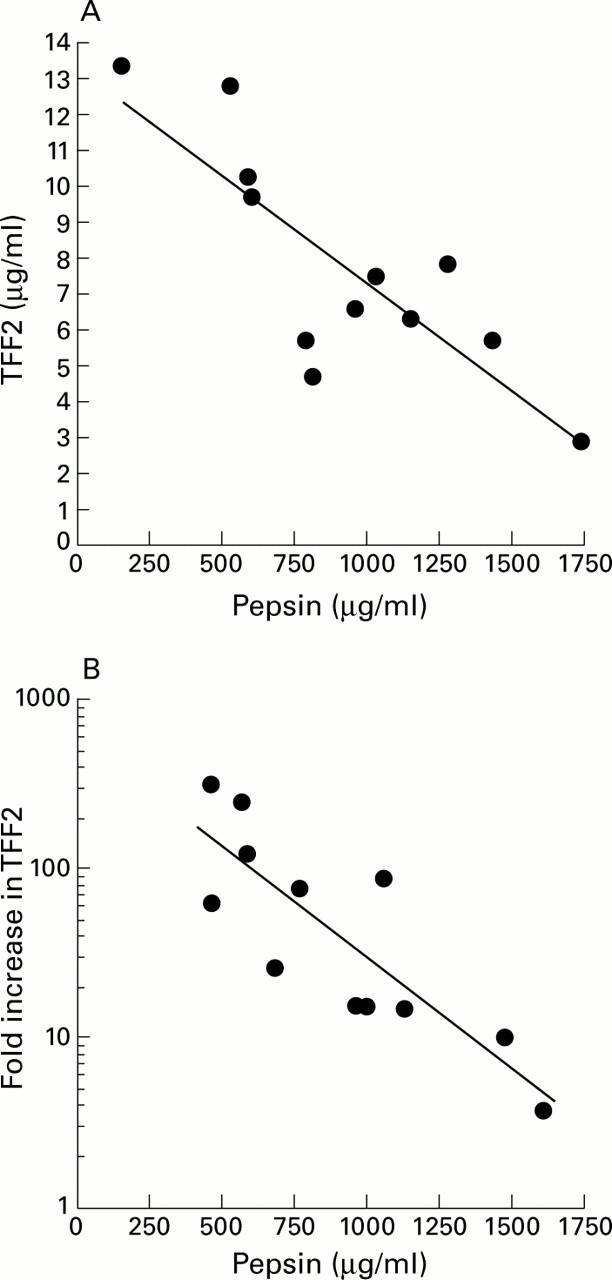
Relationship between pepsin activity and TFF2 concentration in individual gastric juice samples. (A) Gastric juice was collected from 12 young healthy volunteers at 7:00 am. TFF2 concentrations and pepsin activities in gastric juice were measured as described in the methods. Values for TFF2 concentration in gastric juice from each individual are shown plotted against pepsin activity. (B) Gastric juice was collected from 12 young healthy volunteers at two hourly intervals between 7:00 pm and 7:00 am. TFF2 concentrations and pepsin activities in gastric juice were measured as described in the methods. The fold increases in TFF2 concentration over this time were calculated and are plotted against the average pepsin activity for each individual.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alison M. R., Chinery R., Poulsom R., Ashwood P., Longcroft J. M., Wright N. A. Experimental ulceration leads to sequential expression of spasmolytic polypeptide, intestinal trefoil factor, epidermal growth factor and transforming growth factor alpha mRNAs in rat stomach. J Pathol. 1995 Apr;175(4):405–414. doi: 10.1002/path.1711750408. [DOI] [PubMed] [Google Scholar]
- Babyatsky M. W., deBeaumont M., Thim L., Podolsky D. K. Oral trefoil peptides protect against ethanol- and indomethacin-induced gastric injury in rats. Gastroenterology. 1996 Feb;110(2):489–497. doi: 10.1053/gast.1996.v110.pm8566596. [DOI] [PubMed] [Google Scholar]
- Carr M. D., Bauer C. J., Gradwell M. J., Feeney J. Solution structure of a trefoil-motif-containing cell growth factor, porcine spasmolytic protein. Proc Natl Acad Sci U S A. 1994 Mar 15;91(6):2206–2210. doi: 10.1073/pnas.91.6.2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadwick M. P., Westley B. R., May F. E. Homodimerization and hetero-oligomerization of the single-domain trefoil protein pNR-2/pS2 through cysteine 58. Biochem J. 1997 Oct 1;327(Pt 1):117–123. doi: 10.1042/bj3270117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De A., Brown D. G., Gorman M. A., Carr M., Sanderson M. R., Freemont P. S. Crystal structure of a disulfide-linked "trefoil" motif found in a large family of putative growth factors. Proc Natl Acad Sci U S A. 1994 Feb 1;91(3):1084–1088. doi: 10.1073/pnas.91.3.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignass A., Lynch-Devaney K., Kindon H., Thim L., Podolsky D. K. Trefoil peptides promote epithelial migration through a transforming growth factor beta-independent pathway. J Clin Invest. 1994 Jul;94(1):376–383. doi: 10.1172/JCI117332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajhede M., Petersen T. N., Henriksen A., Petersen J. F., Dauter Z., Wilson K. S., Thim L. Pancreatic spasmolytic polypeptide: first three-dimensional structure of a member of the mammalian trefoil family of peptides. Structure. 1993 Dec 15;1(4):253–262. doi: 10.1016/0969-2126(93)90014-8. [DOI] [PubMed] [Google Scholar]
- Hanby A. M., Poulsom R., Singh S., Elia G., Jeffery R. E., Wright N. A. Spasmolytic polypeptide is a major antral peptide: distribution of the trefoil peptides human spasmolytic polypeptide and pS2 in the stomach. Gastroenterology. 1993 Oct;105(4):1110–1116. doi: 10.1016/0016-5085(93)90956-d. [DOI] [PubMed] [Google Scholar]
- Jorde R., Burhol P. G. Diurnal profiles of gastrointestinal regulatory peptides. Scand J Gastroenterol. 1985 Jan;20(1):1–4. doi: 10.3109/00365528509089624. [DOI] [PubMed] [Google Scholar]
- Jørgensen K. D., Diamant B., Jørgensen K. H., Thim L. Pancreatic spasmolytic polypeptide (PSP): III. Pharmacology of a new porcine pancreatic polypeptide with spasmolytic and gastric acid secretion inhibitory effects. Regul Pept. 1982 Mar;3(3-4):231–243. doi: 10.1016/0167-0115(82)90128-8. [DOI] [PubMed] [Google Scholar]
- May F. E., Semple J. I., Newton J. L., Westley B. R. The human two domain trefoil protein, TFF2, is glycosylated in vivo in the stomach. Gut. 2000 Apr;46(4):454–459. doi: 10.1136/gut.46.4.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May F. E., Westley B. R. Close physical linkage of the genes encoding the pNR-2/pS2 protein and human spasmolytic protein (hSP). Hum Genet. 1997 Mar;99(3):303–307. doi: 10.1007/s004390050362. [DOI] [PubMed] [Google Scholar]
- May F. E., Westley B. R. Trefoil proteins: their role in normal and malignant cells. J Pathol. 1997 Sep;183(1):4–7. doi: 10.1002/(SICI)1096-9896(199709)183:1<4::AID-PATH1099>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Ogata H., Podolsky D. K. Trefoil peptide expression and secretion is regulated by neuropeptides and acetylcholine. Am J Physiol. 1997 Aug;273(2 Pt 1):G348–G354. doi: 10.1152/ajpgi.1997.273.2.G348. [DOI] [PubMed] [Google Scholar]
- Piggott N. H., Henry J. A., May F. E., Westley B. R. Antipeptide antibodies against the pNR-2 oestrogen-regulated protein of human breast cancer cells and detection of pNR-2 expression in normal tissues by immunohistochemistry. J Pathol. 1991 Feb;163(2):95–104. doi: 10.1002/path.1711630204. [DOI] [PubMed] [Google Scholar]
- Playford R. J., Marchbank T., Chinery R., Evison R., Pignatelli M., Boulton R. A., Thim L., Hanby A. M. Human spasmolytic polypeptide is a cytoprotective agent that stimulates cell migration. Gastroenterology. 1995 Jan;108(1):108–116. doi: 10.1016/0016-5085(95)90014-4. [DOI] [PubMed] [Google Scholar]
- Polshakov V. I., Williams M. A., Gargaro A. R., Frenkiel T. A., Westley B. R., Chadwick M. P., May F. E., Feeney J. High-resolution solution structure of human pNR-2/pS2: a single trefoil motif protein. J Mol Biol. 1997 Mar 28;267(2):418–432. doi: 10.1006/jmbi.1997.0896. [DOI] [PubMed] [Google Scholar]
- Poulsen S. S., Thulesen J., Christensen L., Nexo E., Thim L. Metabolism of oral trefoil factor 2 (TFF2) and the effect of oral and parenteral TFF2 on gastric and duodenal ulcer healing in the rat. Gut. 1999 Oct;45(4):516–522. doi: 10.1136/gut.45.4.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulsom R. Trefoil peptides. Baillieres Clin Gastroenterol. 1996 Mar;10(1):113–134. doi: 10.1016/s0950-3528(96)90043-3. [DOI] [PubMed] [Google Scholar]
- Rudd P. M., Dwek R. A. Glycosylation: heterogeneity and the 3D structure of proteins. Crit Rev Biochem Mol Biol. 1997;32(1):1–100. doi: 10.3109/10409239709085144. [DOI] [PubMed] [Google Scholar]
- Rudd P. M., Joao H. C., Coghill E., Fiten P., Saunders M. R., Opdenakker G., Dwek R. A. Glycoforms modify the dynamic stability and functional activity of an enzyme. Biochemistry. 1994 Jan 11;33(1):17–22. doi: 10.1021/bi00167a003. [DOI] [PubMed] [Google Scholar]
- Shakin-Eshleman S. H., Spitalnik S. L., Kasturi L. The amino acid at the X position of an Asn-X-Ser sequon is an important determinant of N-linked core-glycosylation efficiency. J Biol Chem. 1996 Mar 15;271(11):6363–6366. doi: 10.1074/jbc.271.11.6363. [DOI] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Svanes C., Sothern R. B., Sørbye H. Rhythmic patterns in incidence of peptic ulcer perforation over 5.5 decades in Norway. Chronobiol Int. 1998 May;15(3):241–264. doi: 10.3109/07420529808998687. [DOI] [PubMed] [Google Scholar]
- Tanaka S., Podolsky D. K., Engel E., Guth P. H., Kaunitz J. D. Human spasmolytic polypeptide decreases proton permeation through gastric mucus in vivo and in vitro. Am J Physiol. 1997 Jun;272(6 Pt 1):G1473–G1480. doi: 10.1152/ajpgi.1997.272.6.G1473. [DOI] [PubMed] [Google Scholar]
- Thim L., Norris K., Norris F., Nielsen P. F., Bjørn S. E., Christensen M., Petersen J. Purification and characterization of the trefoil peptide human spasmolytic polypeptide (hSP) produced in yeast. FEBS Lett. 1993 Mar 8;318(3):345–352. doi: 10.1016/0014-5793(93)80543-4. [DOI] [PubMed] [Google Scholar]
- Thim L. Trefoil peptides: from structure to function. Cell Mol Life Sci. 1997 Dec;53(11-12):888–903. doi: 10.1007/s000180050108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasetto C., Rio M. C., Gautier C., Wolf C., Hareuveni M., Chambon P., Lathe R. hSP, the domain-duplicated homolog of pS2 protein, is co-expressed with pS2 in stomach but not in breast carcinoma. EMBO J. 1990 Feb;9(2):407–414. doi: 10.1002/j.1460-2075.1990.tb08125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong W. M., Poulsom R., Wright N. A. Trefoil peptides. Gut. 1999 Jun;44(6):890–895. doi: 10.1136/gut.44.6.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright N. A., Poulsom R., Stamp G. W., Hall P. A., Jeffery R. E., Longcroft J. M., Rio M. C., Tomasetto C., Chambon P. Epidermal growth factor (EGF/URO) induces expression of regulatory peptides in damaged human gastrointestinal tissues. J Pathol. 1990 Dec;162(4):279–284. doi: 10.1002/path.1711620402. [DOI] [PubMed] [Google Scholar]
- Wright N. A., Poulsom R., Stamp G., Van Noorden S., Sarraf C., Elia G., Ahnen D., Jeffery R., Longcroft J., Pike C. Trefoil peptide gene expression in gastrointestinal epithelial cells in inflammatory bowel disease. Gastroenterology. 1993 Jan;104(1):12–20. doi: 10.1016/0016-5085(93)90830-6. [DOI] [PubMed] [Google Scholar]