Full Text
The Full Text of this article is available as a PDF (94.4 KB).
Figure 1 .
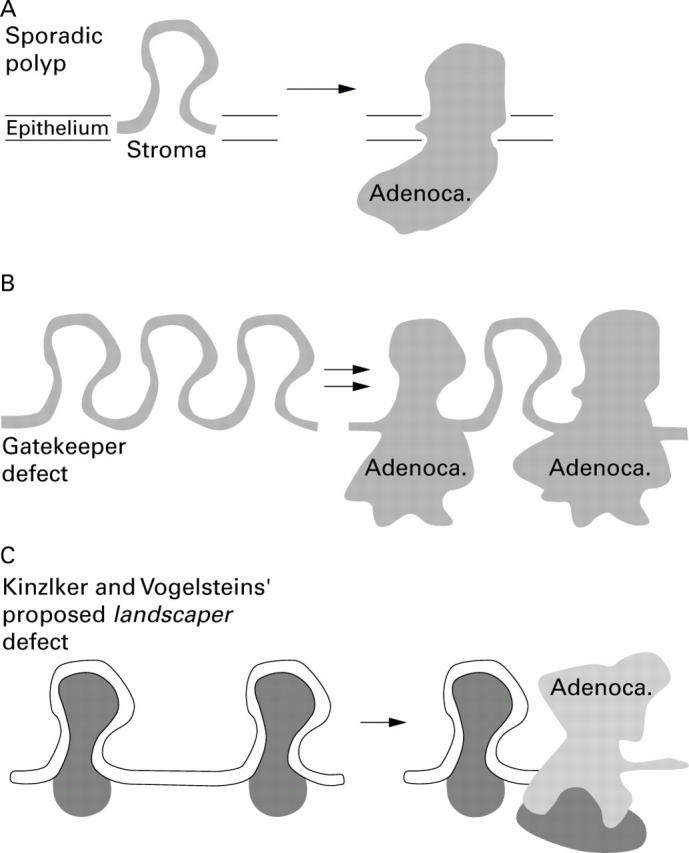
Schematic representation of colonic mucosa, divided into stroma and overlying epithelium. Regions with functionally relevant genetic defects are shown in light and dark grey. Adenoca., adenocarcinoma. (A) Sporadic colonic tumours acquire initial functional genetic defects in specific regions of the epithelium, causing dysplastic polyps that may progress to carcinoma. (B) In patients with conditions such as familial adenomatous polyposis, the whole of the epithelium is affected by a defect in a tumour suppressor gene, resulting in the development of multiple dysplastic polyps. Some of these polyps will subsequently progress to carcinoma. A defect in a tumour suppressor gene resulting in multiple polyps can be considered a loss of control of gatekeeper function. (C) Patients with juvenile polyposis syndrome (JPS) initially develop hamartomas that were thought to have functional genetic defects (that is, loss of the second functional allele of SMAD4) only affecting the stroma. The overlying epithelium was considered to be still under functional genetic control of the remaining normal SMAD4 allele. According to the landscaper hypothesis, this abnormality of the stroma subsequently induced the overlying epithelium to progress to carcinoma. The recent paper by Woodford-Richens et al casts doubt on the relevance of this model in patients with JPS as they have identical genetic defects affecting the overlying epithelium.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abeln E. C., Smit V. T., Wessels J. W., de Leeuw W. J., Cornelisse C. J., Fleuren G. J. Molecular genetic evidence for the conversion hypothesis of the origin of malignant mixed müllerian tumours. J Pathol. 1997 Dec;183(4):424–431. doi: 10.1002/(SICI)1096-9896(199712)183:4<424::AID-PATH949>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Green A. J., Sepp T., Yates J. R. Clonality of tuberous sclerosis harmatomas shown by non-random X-chromosome inactivation. Hum Genet. 1996 Feb;97(2):240–243. doi: 10.1007/BF02265273. [DOI] [PubMed] [Google Scholar]
- Gruber S. B., Entius M. M., Petersen G. M., Laken S. J., Longo P. A., Boyer R., Levin A. M., Mujumdar U. J., Trent J. M., Kinzler K. W. Pathogenesis of adenocarcinoma in Peutz-Jeghers syndrome. Cancer Res. 1998 Dec 1;58(23):5267–5270. [PubMed] [Google Scholar]
- Hemminki A., Tomlinson I., Markie D., Järvinen H., Sistonen P., Björkqvist A. M., Knuutila S., Salovaara R., Bodmer W., Shibata D. Localization of a susceptibility locus for Peutz-Jeghers syndrome to 19p using comparative genomic hybridization and targeted linkage analysis. Nat Genet. 1997 Jan;15(1):87–90. doi: 10.1038/ng0197-87. [DOI] [PubMed] [Google Scholar]
- Houlston R., Bevan S., Williams A., Young J., Dunlop M., Rozen P., Eng C., Markie D., Woodford-Richens K., Rodriguez-Bigas M. A. Mutations in DPC4 (SMAD4) cause juvenile polyposis syndrome, but only account for a minority of cases. Hum Mol Genet. 1998 Nov;7(12):1907–1912. doi: 10.1093/hmg/7.12.1907. [DOI] [PubMed] [Google Scholar]
- Howe J. R., Roth S., Ringold J. C., Summers R. W., Järvinen H. J., Sistonen P., Tomlinson I. P., Houlston R. S., Bevan S., Mitros F. A. Mutations in the SMAD4/DPC4 gene in juvenile polyposis. Science. 1998 May 15;280(5366):1086–1088. doi: 10.1126/science.280.5366.1086. [DOI] [PubMed] [Google Scholar]
- Jacoby R. F., Schlack S., Cole C. E., Skarbek M., Harris C., Meisner L. F. A juvenile polyposis tumor suppressor locus at 10q22 is deleted from nonepithelial cells in the lamina propria. Gastroenterology. 1997 Apr;112(4):1398–1403. doi: 10.1016/s0016-5085(97)70156-2. [DOI] [PubMed] [Google Scholar]
- Kinzler K. W., Vogelstein B. Landscaping the cancer terrain. Science. 1998 May 15;280(5366):1036–1037. doi: 10.1126/science.280.5366.1036. [DOI] [PubMed] [Google Scholar]
- Lazutka J. R. Genetic toxicity of cytokines. Mutat Res. 1996 Dec 12;361(2-3):95–105. doi: 10.1016/s0165-1161(96)00027-1. [DOI] [PubMed] [Google Scholar]
- Marsh D. J., Dahia P. L., Coulon V., Zheng Z., Dorion-Bonnet F., Call K. M., Little R., Lin A. Y., Eeles R. A., Goldstein A. M. Allelic imbalance, including deletion of PTEN/MMACI, at the Cowden disease locus on 10q22-23, in hamartomas from patients with Cowden syndrome and germline PTEN mutation. Genes Chromosomes Cancer. 1998 Jan;21(1):61–69. doi: 10.1002/(sici)1098-2264(199801)21:1<61::aid-gcc8>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Moskaluk C. A., Hruban R. H., Schutte M., Lietman A. S., Smyrk T., Fusaro L., Fusaro R., Lynch J., Yeo C. J., Jackson C. E. Genomic sequencing of DPC4 in the analysis of familial pancreatic carcinoma. Diagn Mol Pathol. 1997 Apr;6(2):85–90. doi: 10.1097/00019606-199704000-00003. [DOI] [PubMed] [Google Scholar]
- Takagi Y., Kohmura H., Futamura M., Kida H., Tanemura H., Shimokawa K., Saji S. Somatic alterations of the DPC4 gene in human colorectal cancers in vivo. Gastroenterology. 1996 Nov;111(5):1369–1372. doi: 10.1053/gast.1996.v111.pm8898652. [DOI] [PubMed] [Google Scholar]
- Tamir S., deRojas-Walker T., Wishnok J. S., Tannenbaum S. R. DNA damage and genotoxicity by nitric oxide. Methods Enzymol. 1996;269:230–243. doi: 10.1016/s0076-6879(96)69025-9. [DOI] [PubMed] [Google Scholar]
- Veale A. M., McColl I., Bussey H. J., Morson B. C. Juvenile polyposis coli. J Med Genet. 1966 Mar;3(1):5–16. doi: 10.1136/jmg.3.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel G. Can old cells learn new tricks? Science. 2000 Feb 25;287(5457):1418–1419. doi: 10.1126/science.287.5457.1418. [DOI] [PubMed] [Google Scholar]
- Wang Z. J., Ellis I., Zauber P., Iwama T., Marchese C., Talbot I., Xue W. H., Yan Z. Y., Tomlinson I. Allelic imbalance at the LKB1 (STK11) locus in tumours from patients with Peutz-Jeghers' syndrome provides evidence for a hamartoma-(adenoma)-carcinoma sequence. J Pathol. 1999 May;188(1):9–13. doi: 10.1002/(SICI)1096-9896(199905)188:1<9::AID-PATH326>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Woodford-Richens K., Williamson J., Bevan S., Young J., Leggett B., Frayling I., Thway Y., Hodgson S., Kim J. C., Iwama T. Allelic loss at SMAD4 in polyps from juvenile polyposis patients and use of fluorescence in situ hybridization to demonstrate clonal origin of the epithelium. Cancer Res. 2000 May 1;60(9):2477–2482. [PubMed] [Google Scholar]
- Yasunaga Y., Shinomura Y., Kanayama S., Higashimoto Y., Yabu M., Miyazaki Y., Kondo S., Murayama Y., Nishibayashi H., Kitamura S. Increased production of interleukin 1 beta and hepatocyte growth factor may contribute to foveolar hyperplasia in enlarged fold gastritis. Gut. 1996 Dec;39(6):787–794. doi: 10.1136/gut.39.6.787. [DOI] [PMC free article] [PubMed] [Google Scholar]


