Abstract
BACKGROUND—Neuroendocrine cell (NEC) carcinoma is occasionally accompanied by adenocarcinoma but the relationship between these two morphologically distinct tumours is unclear. Two hypotheses have arisen regarding the mechanism for the association of adenocarcinoma and NEC carcinoma. One is that both are derived from a common multipotential epithelial stem cell. The second hypothesis is that adenocarcinoma and NEC carcinoma arise from a multipotential epithelial stem cell and a primitive NEC, respectively. AIMS—To elucidate the relationship between the two histologically distinct tumours, adenocarcinoma of the stomach and NEC carcinoma of the duodenum. PATIENT/METHODS—We present a case in which the tumour extended across the pyloric ring, the gastric portion of which revealed adenocarcinoma while the duodenal portion showed argyrophil NEC carcinoma. The two histologically distinct lesions of the tumour were examined by immunohistochemistry and genetic analysis of p53. RESULTS—The gastric region was negative for chromogranin A staining but positive for carcinoembryonic antigen (CEA) staining. In contrast, the duodenal region was positive for chromogranin A but negative for CEA. All tumour regions showed a point mutation in p53 gene at exon 7 (GGC (glycine)→GTC (valine) at codon 245). The distal portion of the duodenal tumour showed an additional point mutation in p53 gene at exon 5 (GCC (alanine)→GTC (valine) at codon 129). CONCLUSIONS—The two histologically distinct tumours, adenocarcinoma of the stomach and NEC carcinoma of the duodenum, appear to be derived from a common epithelial cell. Keywords: neuroendocrine cell carcinomas; adenocarcinoma; composite tumour; collision tumours
Full Text
The Full Text of this article is available as a PDF (152.8 KB).
Figure 1 .
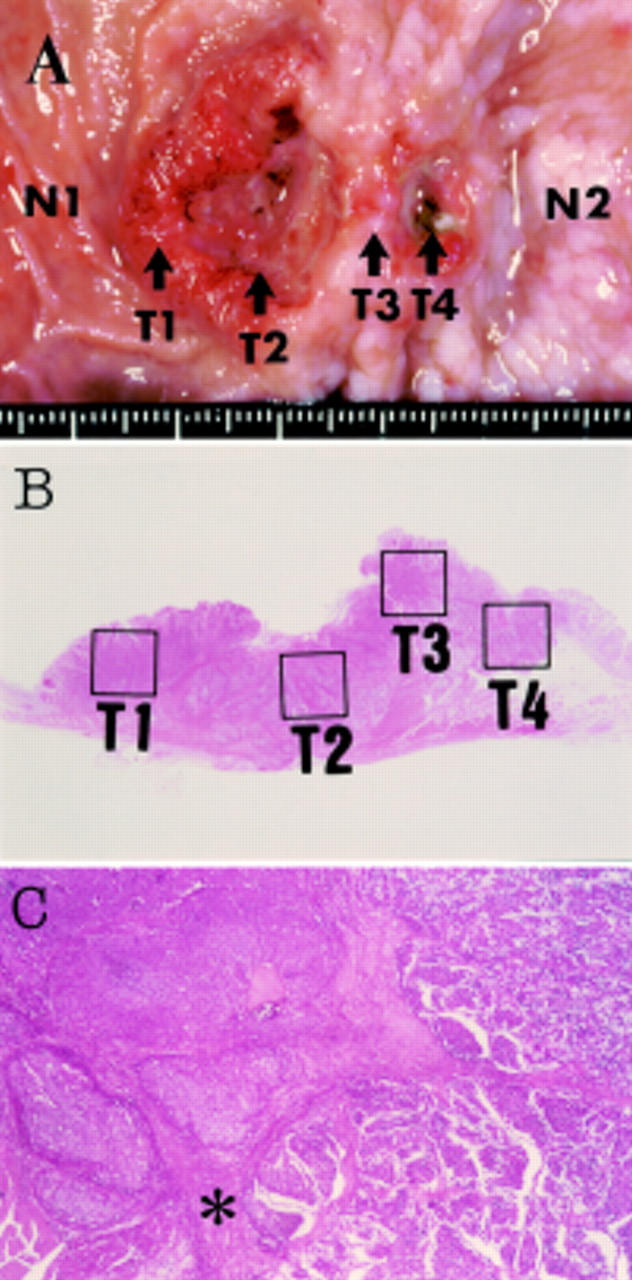
(A) Macroscopic view of the resected specimen showing a spherical tumour located at the pre-pylorus and duodenum. Tissues from six parts in and around the gastroduodenal carcinoma were microdissected to analyse p53 gene mutations: normal duodenum (N1), anal side of duodenal tumour (T1), centre of duodenal tumour (T2), tumour at the pyloric ring (T3), tumour of the stomach (T4), and normal stomach (N2). (B) Distinct areas (T1-T4) which were analysed for p53 gene mutation are indicated by the solid lines. (C) The well circumscribed tumour cell nests in the duodenal region revealed typical medullar growth of neuroendocrine carcinoma (left side). Poorly differentiated adenocarcinoma in the stomach showed small acinar pattern (right side). The pyloric sphincter (asterisk) clearly divides the two histologically distinct lesions.
Figure 2 .
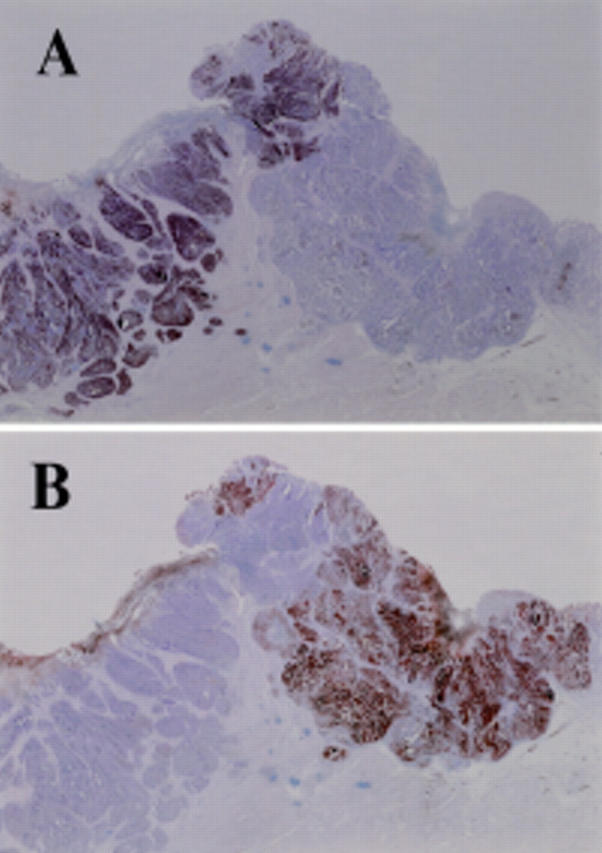
Immunohistochemical staining of the gastroduodenal tumour. (A) The duodenal lesion (neuroendocrine cell carcinoma) was positive for chromogranin A but the gastric lesion (adenocarcinoma) gave a negative result. (B) In contrast, the gastric lesion was positive for carcinoembryonic antigen but the duodenal lesion was negative.
Figure 3 .
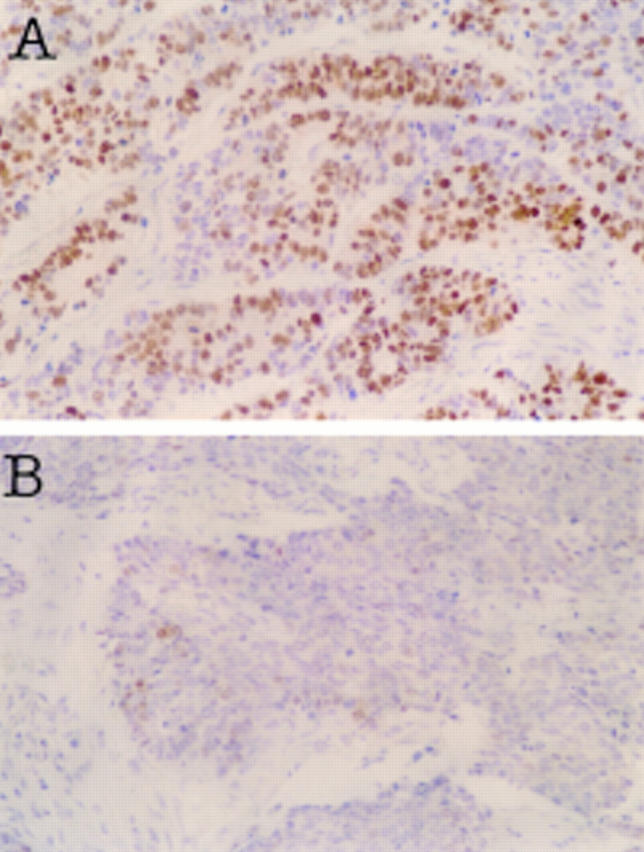
Immunohistochemical staining for p53 protein. (A) Strong nuclear accumulation of the p53 protein in most of the tumour cells in the adenocarcinoma component was observed. (B) Only a few cells in the neuroendocrine cell (NEC) carcinoma region were positive for p53 protein. There was no difference in p53 staining pattern in the different portion (T1, T2, and T3) of the NEC carcinoma (data not shown).


