Abstract
Conjugated linoleic acid (CLA) is a collective term referring to the positional and geometric isomers of linoleic acid. This novel fatty acid has been shown to have a number of beneficial actions, including immunomodulatory, anticarcinogenic, and antiatherogenic effects. Tight junctions of epithelial cells determine epithelial membrane integrity and selective paracellular permeability to ions and macromolecules. Occludin and ZO-1 are integral structural components of the tight junction, which are involved in the biogenesis and functional integrity of the epithelial monolayer. This study investigated the effects of two isomers of CLA (cis-9 and trans-10 isomers) on Caco-2 cell transepithelial resistance (TER) development, paracellular epithelial permeability, and occludin and ZO-1 expression. Caco-2 cells were grown in media supplemented with 0.05 mM linoleic acid, cis-9 CLA, or trans-10 CLA for 21 days. The trans-10 CLA isomer delayed Caco-2 cell TER development, which is an in vitro measure of epithelial cell integrity, and increased paracellular epithelial permeability. Immunofluorescent staining of Caco-2 cell epithelial monolayers grown in media supplemented trans-10 CLA showed that the trans-10 CLA isomer altered distribution of occludin and ZO-1. The trans-10 CLA isomer delayed the acquisition of transepithelial resistance and altered the cellular distribution of occludin, which have important implications in relation to epithelial permeability. Keywords: conjugated linoleic acid; occludin; ZO-1; tight junction; epithelial permeability; Caco-2 cells
Full Text
The Full Text of this article is available as a PDF (233.3 KB).
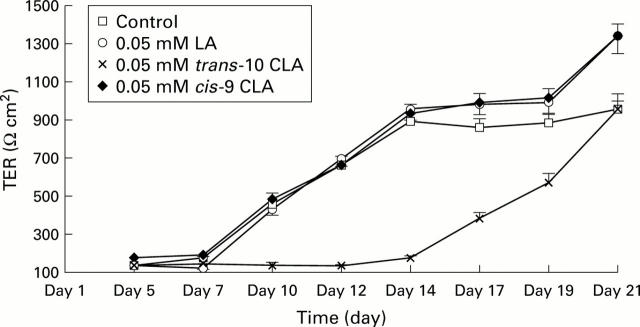
Figure 1 .
Transenodothelial cell resistance (TER) of cells grown in media supplemented with linoleic acid (LA), trans-10 conjugated linoleic acid (CLA), and cis-9 CLA (0.05 mM) compared with non-supplemented media (controls) (passage 14: 130 cells/µl). Values are mean (SEM) TER values of three wells per fatty acid treatment.
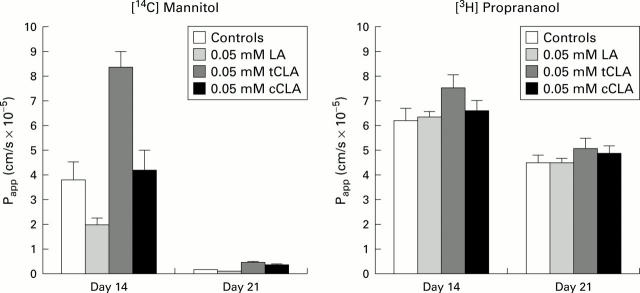
Figure 2 .
Mean transfer (DPM/hour) of [14C] mannitol and [3H] proprananol of control cells and cells supplemented with 0.05 mM linoleic acid (LA), trans-10 conjugated linoleic acid (tCLA), and cis-9 CLA (cCLA) for day 14 and day 21 (passage 13: 200 cells/µl). Values are mean (SEM) of three wells per fatty acid treatment.
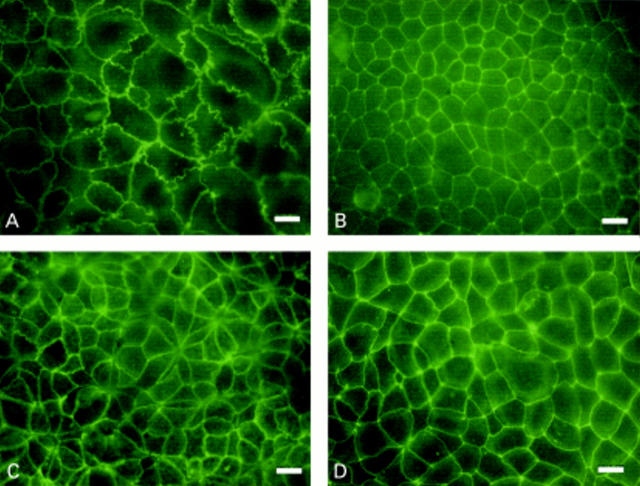
Figure 3 .
Antioccludin immunofluorescent staining of Caco-2 cell monolayers grown in media supplemented with 0.05 mM linoleic acid (LA) (A), trans-10 conjugated linoleic acid (CLA) (B), and cis-9 CLA (C) for 14 days. Bar 40 µm.
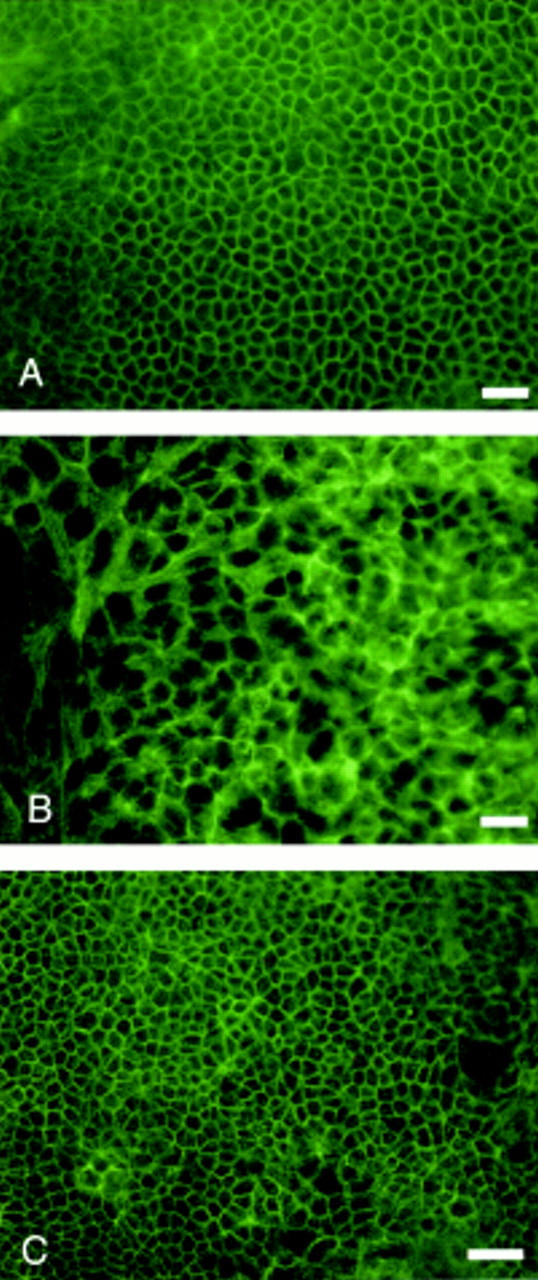
Figure 4 .
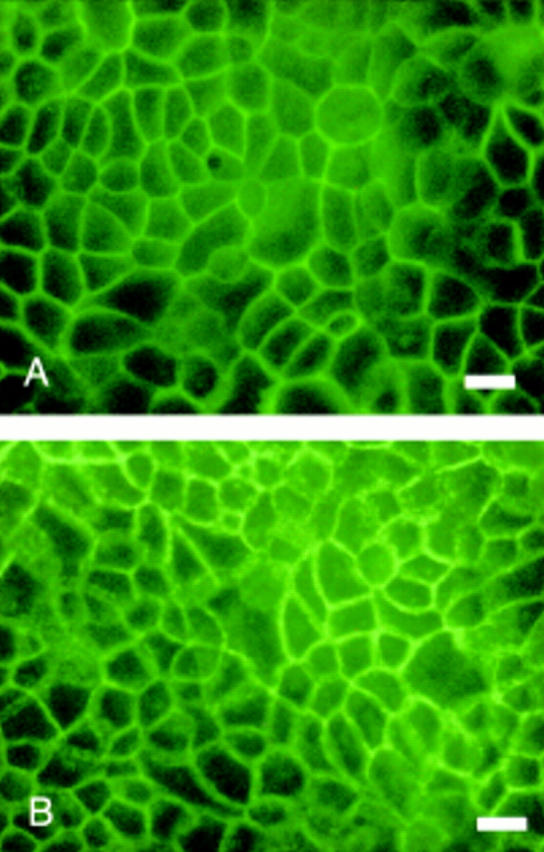
Antioccludin immunofluorescent staining of Caco-2 cell monolayers grown in media supplemented with 0.05 mM linoleic acid (LA) (A) and trans-10 conjugated linoleic acid (CLA) (B) for 14 days. Bar 100 µm
Figure 5 .
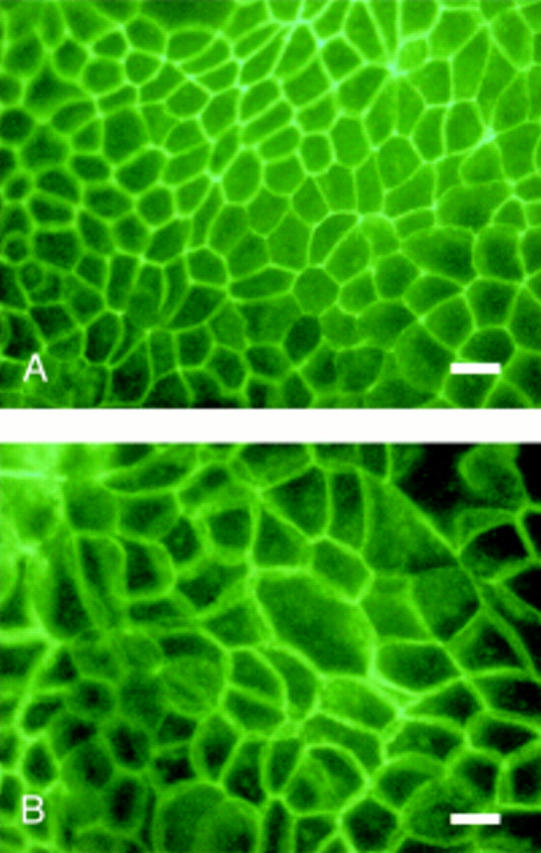
Antioccludin immunofluorescent staining of Caco-2 cell monolayers grown in media supplemented with 0.05 mM linoleic acid (LA) (A) and trans-10 conjugated linoleic acid (CLA) (B) for 21 days. Bar 100 µm
Figure 6 .
Anti-ZO-1 immunofluorescent staining of Caco-2 cell monolayers grown in media supplemented with 0.05 mM trans-10 conjugated linoleic acid (CLA) (A, B) and cis-9 CLA (C, D) for 14 and 21 days, respectively. Bar 100 µm
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. M., Balda M. S., Fanning A. S. The structure and regulation of tight junctions. Curr Opin Cell Biol. 1993 Oct;5(5):772–778. doi: 10.1016/0955-0674(93)90024-k. [DOI] [PubMed] [Google Scholar]
- Anderson J. M., Van Itallie C. M. Tight junctions and the molecular basis for regulation of paracellular permeability. Am J Physiol. 1995 Oct;269(4 Pt 1):G467–G475. doi: 10.1152/ajpgi.1995.269.4.G467. [DOI] [PubMed] [Google Scholar]
- Balda M. S., Gonzalez-Mariscal L., Matter K., Cereijido M., Anderson J. M. Assembly of the tight junction: the role of diacylglycerol. J Cell Biol. 1993 Oct;123(2):293–302. doi: 10.1083/jcb.123.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balda M. S., Matter K. Tight junctions. J Cell Sci. 1998 Mar;111(Pt 5):541–547. doi: 10.1242/jcs.111.5.541. [DOI] [PubMed] [Google Scholar]
- Balda M. S., Whitney J. A., Flores C., González S., Cereijido M., Matter K. Functional dissociation of paracellular permeability and transepithelial electrical resistance and disruption of the apical-basolateral intramembrane diffusion barrier by expression of a mutant tight junction membrane protein. J Cell Biol. 1996 Aug;134(4):1031–1049. doi: 10.1083/jcb.134.4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew B. P., Wong T. S., Shultz T. D., Magnuson N. S. Effects of conjugated dienoic derivatives of linoleic acid and beta-carotene in modulating lymphocyte and macrophage function. Anticancer Res. 1997 Mar-Apr;17(2A):1099–1106. [PubMed] [Google Scholar]
- Furuse M., Itoh M., Hirase T., Nagafuchi A., Yonemura S., Tsukita S., Tsukita S. Direct association of occludin with ZO-1 and its possible involvement in the localization of occludin at tight junctions. J Cell Biol. 1994 Dec;127(6 Pt 1):1617–1626. doi: 10.1083/jcb.127.6.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirase T., Staddon J. M., Saitou M., Ando-Akatsuka Y., Itoh M., Furuse M., Fujimoto K., Tsukita S., Rubin L. L. Occludin as a possible determinant of tight junction permeability in endothelial cells. J Cell Sci. 1997 Jul;110(Pt 14):1603–1613. doi: 10.1242/jcs.110.14.1603. [DOI] [PubMed] [Google Scholar]
- Jiang W. G., Bryce R. P., Horrobin D. F., Mansel R. E. Regulation of tight junction permeability and occludin expression by polyunsaturated fatty acids. Biochem Biophys Res Commun. 1998 Mar 17;244(2):414–420. doi: 10.1006/bbrc.1998.8288. [DOI] [PubMed] [Google Scholar]
- Kim S. K. Tight junctions, membrane-associated guanylate kinases and cell signaling. Curr Opin Cell Biol. 1995 Oct;7(5):641–649. doi: 10.1016/0955-0674(95)80105-7. [DOI] [PubMed] [Google Scholar]
- Lee K. N., Kritchevsky D., Pariza M. W. Conjugated linoleic acid and atherosclerosis in rabbits. Atherosclerosis. 1994 Jul;108(1):19–25. doi: 10.1016/0021-9150(94)90034-5. [DOI] [PubMed] [Google Scholar]
- Lin H., Boylston T. D., Chang M. J., Luedecke L. O., Shultz T. D. Survey of the conjugated linoleic acid contents of dairy products. J Dairy Sci. 1995 Nov;78(11):2358–2365. doi: 10.3168/jds.s0022-0302(95)76863-1. [DOI] [PubMed] [Google Scholar]
- Lindmark T., Kimura Y., Artursson P. Absorption enhancement through intracellular regulation of tight junction permeability by medium chain fatty acids in Caco-2 cells. J Pharmacol Exp Ther. 1998 Jan;284(1):362–369. [PubMed] [Google Scholar]
- Nicolosi R. J., Rogers E. J., Kritchevsky D., Scimeca J. A., Huth P. J. Dietary conjugated linoleic acid reduces plasma lipoproteins and early aortic atherosclerosis in hypercholesterolemic hamsters. Artery. 1997;22(5):266–277. [PubMed] [Google Scholar]
- Parkos C. A., Colgan S. P., Delp C., Arnaout M. A., Madara J. L. Neutrophil migration across a cultured epithelial monolayer elicits a biphasic resistance response representing sequential effects on transcellular and paracellular pathways. J Cell Biol. 1992 May;117(4):757–764. doi: 10.1083/jcb.117.4.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubas W., Cromwell M. E., Mrsny R. J., Ingle G., Elias K. A. An integrated method to determine epithelial transport and bioactivity of oral drug candidates in vitro. Pharm Res. 1996 Jan;13(1):23–26. doi: 10.1023/a:1016060729594. [DOI] [PubMed] [Google Scholar]
- Stuart R. O., Nigam S. K. Regulated assembly of tight junctions by protein kinase C. Proc Natl Acad Sci U S A. 1995 Jun 20;92(13):6072–6076. doi: 10.1073/pnas.92.13.6072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visonneau S., Cesano A., Tepper S. A., Scimeca J. A., Santoli D., Kritchevsky D. Conjugated linoleic acid suppresses the growth of human breast adenocarcinoma cells in SCID mice. Anticancer Res. 1997 Mar-Apr;17(2A):969–973. [PubMed] [Google Scholar]
- Wong V. Phosphorylation of occludin correlates with occludin localization and function at the tight junction. Am J Physiol. 1997 Dec;273(6 Pt 1):C1859–C1867. doi: 10.1152/ajpcell.1997.273.6.C1859. [DOI] [PubMed] [Google Scholar]