Abstract
BACKGROUND AND AIMS—Body gastritis caused by Helicobacter pylori infection appears to inhibit gastric acid secretion. The aim of this study was to determine the effects of H pylori infection on gastric acid secretion and clarify its mechanisms with reference to interleukin 1β (IL-1β). METHODS—(1) Mongolian gerbils were inoculated orally with H pylori. Before, six, and 12 weeks after inoculation, serum gastrin levels, gastric acid output, and IL-1β mRNA levels in the gastric mucosa were determined. Pathological changes were also determined according to the updated Sydney system. (2) Effects of recombinant human IL-1 receptor antagonist (rhIL-1ra) on gastric acid output and serum gastrin levels were also determined. RESULTS—(1) Scores for activity and inflammation of gastritis and serum gastrin levels were significantly increased, and gastric acid output was significantly decreased six and 12 weeks after inoculation with H pylori. IL-1β mRNA levels in the gastric mucosa were also elevated six and 12 weeks after inoculation with H pylori. (2) Acid output and serum gastrin levels in the infected groups returned to control levels after rhIL-1ra injection. CONCLUSIONS—Gastric acid secretion is decreased and serum gastrin levels are increased in Mongolian gerbils infected with H pylori. This change in gastric acid secretion appears to be mediated by IL-1β induced by H pylori infection. Keywords: Helicobacter pylori; interleukin 1β; Mongolian gerbils; gastric acid; gastrin
Full Text
The Full Text of this article is available as a PDF (303.0 KB).
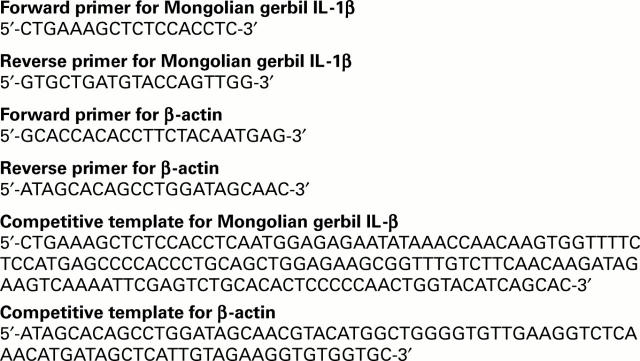
Figure 1 .
Oligonucleotide sequences of polymerase chain reaction (PCR) primers and competitive templates. The primer pair for Mongolian gerbil interleukin 1β (IL-1β) amplifies a 296 bp band. The primer pair for β-actin amplifies a 164 bp band. The competitive template for Mongolian gerbil IL-1β is 150 bp long, and that for β-actin is 82 bp long. PCR products of cDNA are distinguished by size from those of competitors.
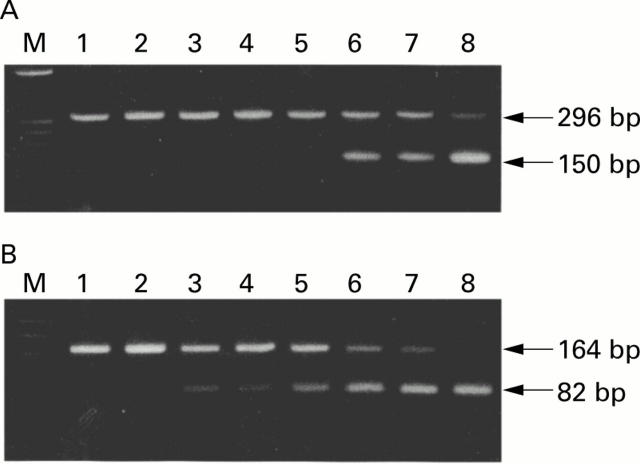
Figure 2 .
(A) Mongolian gerbil interleukin 1β (IL-1β) (296 bp) versus competitor (150 bp). Lane M is the size marker (øX174 digested with Hae III). Lanes 1-8 contain eightfold serial amounts of competitor DNA ranging from 1.0×10-0.5 pg/µl to 1.0×103 pg/µl competing against a fixed dose of cDNA. (B) β-actin (164 bp) versus competitor (82 bp). Lane M is the size marker (øX174 digested with Hinf I). Lanes 1-8 contain eightfold serial amounts of competitor DNA ranging from 1.0×10-0.5 pg/µl to 1.0×103 pg/µl competing against a fixed dose of cDNA.
Figure 3 .
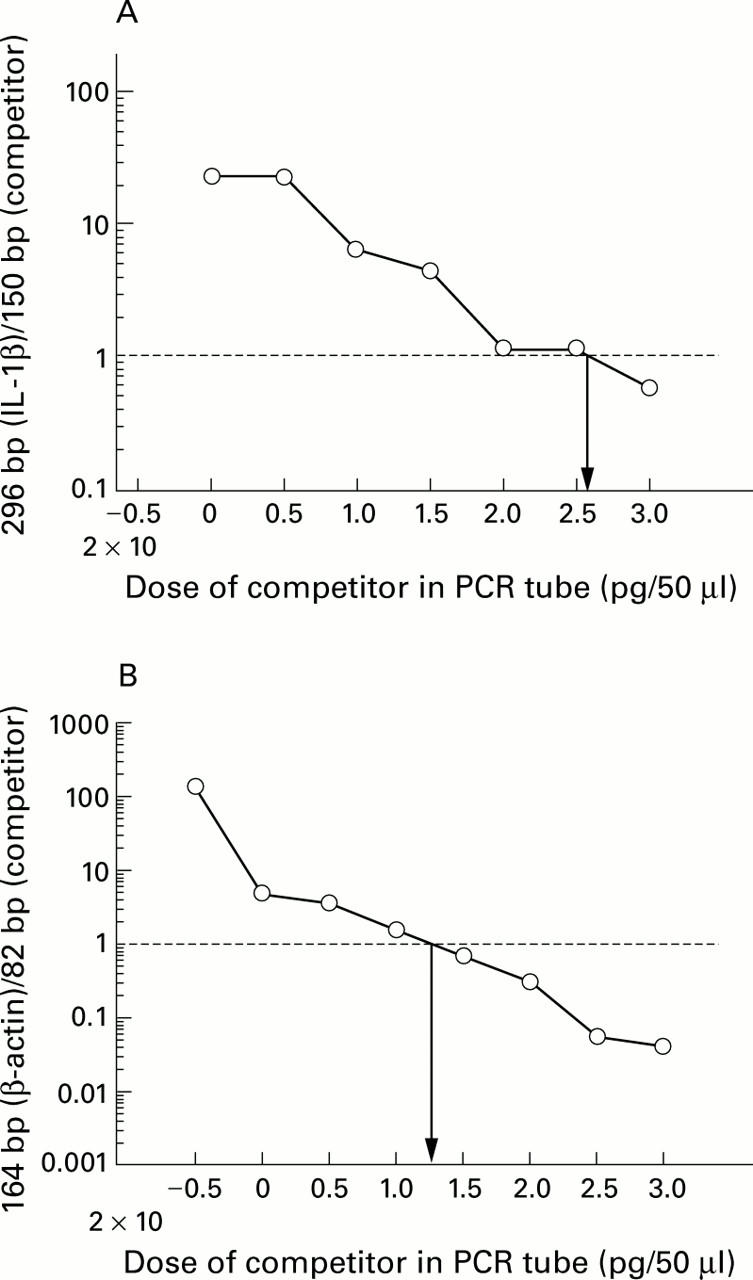
(A) Logarithmic plot of the ratio of Mongolian gerbil interleukin 1β (IL-1β, 296 bp) to competitor (150 bp), corresponding to fig 2A. The quantity of IL-1β mRNA in the reaction tube was determined by calculating how much of the competitor was required to achieve equal amounts of product. The amount of IL-1β mRNA was then calculated by extrapolating from the intersection of the curves to the x axis where the amounts of IL-1β (296 bp) and competitor were equal. (B) Logarithmic plot of the ratio of β-actin (164 bp) to competitor (82 bp), corresponding to fig 2B.
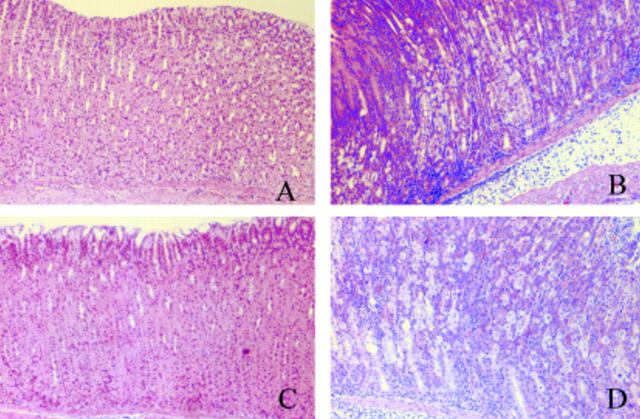
Figure 4 .
Histopathological changes in the gastric mucosa of Mongolian gerbils. (A) Inflammatory cell infiltrations were not observed in the control groups six weeks after inoculation. (B) Marked inflammatory cell infiltrations were observed in the infected groups six weeks after inoculation with H pylori. (C) Inflammatory cell infiltrations were not observed in the control groups 12 weeks after inoculation. (D) Severe inflammatory cell infiltrations were observed in the infected groups 12 weeks after inoculation with H pylori (haematoxylin-eosin, ×100).
Figure 5 .
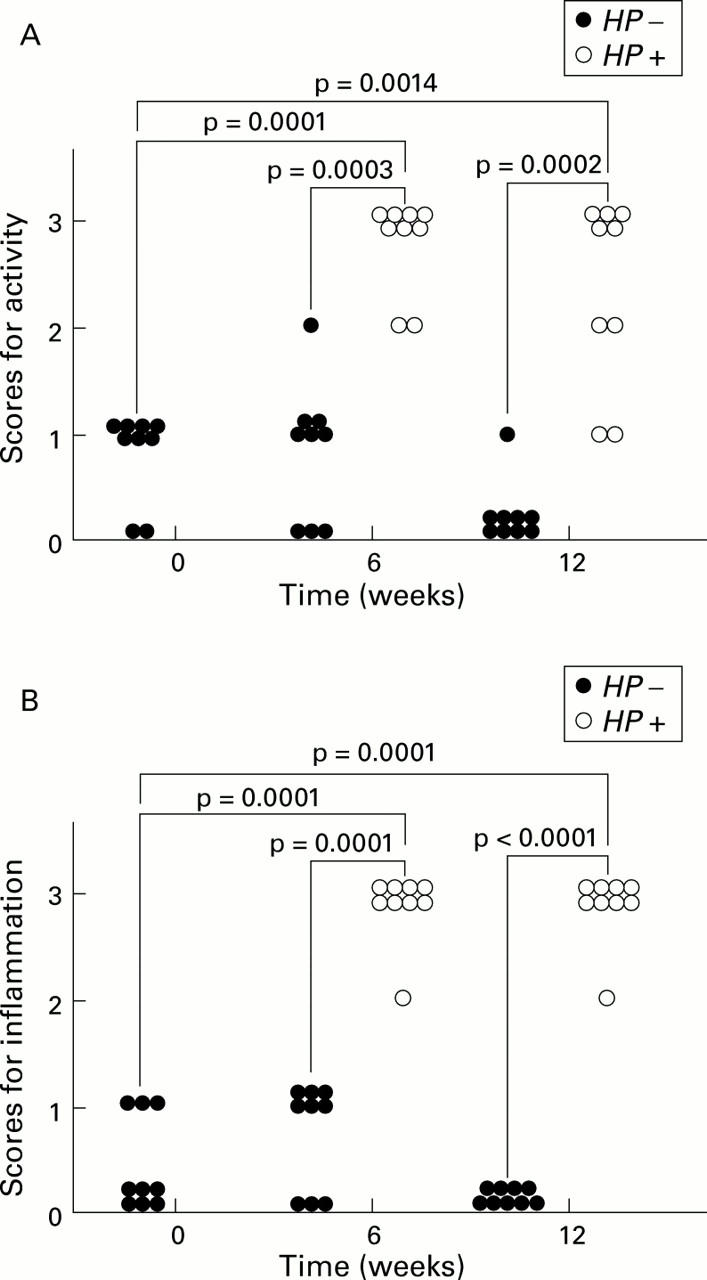
(A) Changes in gastritis scores for activity from before to six and 12 weeks after inoculation in the infected (Helicobacter pylori (HP+)) and control (HP−) groups. The variables used for grading gastritis were rated on a four point scale: 0, absent; 1, mild; 2, moderate; and 3, severe, in accordance with the Sydney system. Gastritis scores for activity in the infected groups significantly increased six and 12 weeks after H pylori inoculation. No significant changes were observed in the control groups. (B) Changes in gastritis scores for inflammation from before to six and 12 weeks after inoculation in the infected and control groups. Gastritis scores for inflammation in the infected groups significantly increased six and 12 weeks after H pylori inoculation. No significant changes were observed in the control groups.
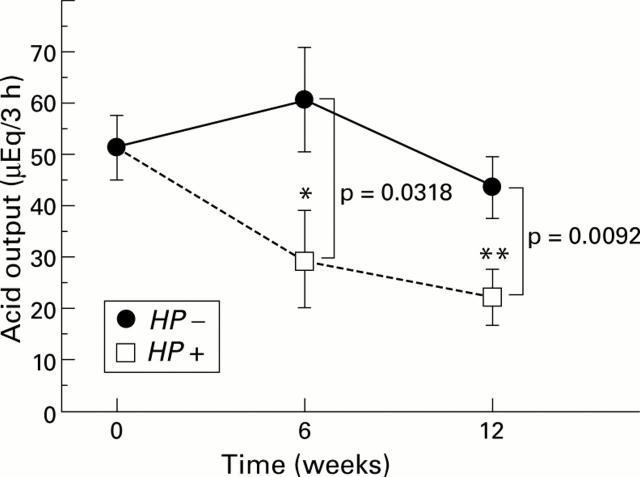
Figure 6 .
Changes in gastric acid output from before to six and 12 weeks after inoculation in the infected (Helicobacter pylori (HP+)) and control (HP−) groups. Gastric acid output in the infected groups significantly decreased six and 12 weeks after H pylori inoculation. No significant changes were observed in the control groups. *p=0.0328; **p=0.0061 compared with before inoculation.
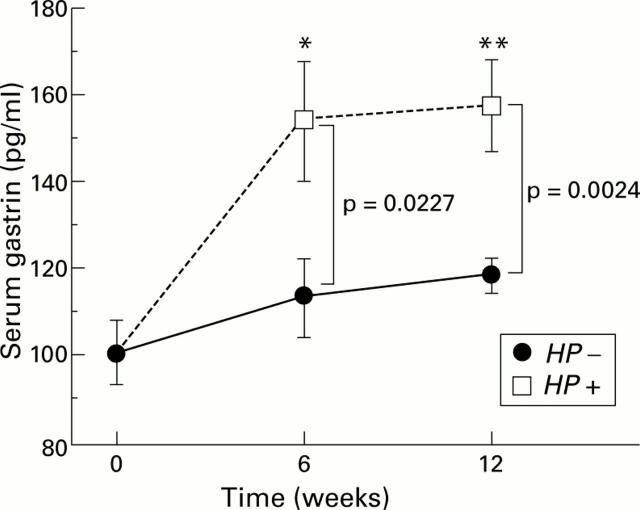
Figure 7 .
Changes in serum gastrin levels from before to 6 and 12 weeks after inoculation. Serum gastrin levels in the infected (Helicobacter pylori (HP+)) groups significantly increased six and 12 weeks after H pylori inoculation. No significant changes were observed in the control groups (HP−). *p=0.0014; **p=0.0008 compared with before innoculation.
Figure 8 .

(A) Alignment of the 296 bp portion of interleukin 1β (IL-1β) cDNA sequences for the Mongolian gerbil, human, and mouse. Oligonucleotides in bold type in human and mouse are different from those of Mongolian gerbil; 85.1% oligonucleotide identities were observed between Mongolian gerbil and human and 90.2% between Mongolian gerbil and mouse. (B) Alignment of the 164 bp portion of β-actin cDNA sequences for the three species. Oligonucleotides in bold type in human and mouse were different from those of the Mongolian gerbil; 97.0% oligonucleotide identities were observed between Mongolian gerbil and human and 96.3% between Mongolian gerbil and mouse.
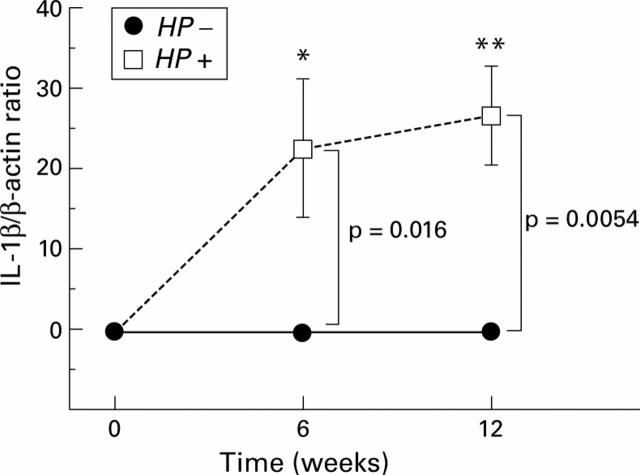
Figure 9 .
Changes in interleukin 1β (IL-1β) mRNA levels from before to six and 12 weeks after inoculation in the infected (Helicobacter pylori (HP+)) and control (HP−) groups. Il-1β mRNA levels significantly increased six and 12 weeks after H pylori inoculation. IL-1β mRNA was virtually undetected in the control groups throughout the study. *p=0.0191; **p=0.0006 compared with before innoculation.
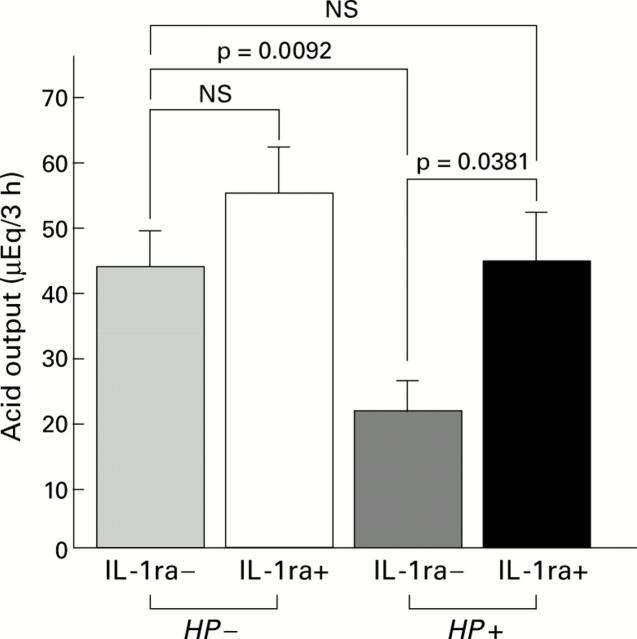
Figure 10 .
Gastric acid output with (+) or without (−) recombinant human IL-1 receptor antagonist (rhIL-1ra) injection in the infected (Helicobacter pylori (HP+)) and control (HP−) groups. Gastric acid output significantly increased to control levels after rhIL-1ra injection in the infected groups whereas no significant changes were observed after rhIL-1ra injection in the control groups.
Figure 11 .
Serum gastrin levels with (+) or without (−) recombinant human IL-1 receptor antagonist (rhIL-1ra) injection in the infected (Helicobacter pylori (HP+)) and control (HP−) groups. Serum gastrin levels significantly decreased to control levels after rhIL-1ra injection in the infected groups whereas no significant changes were observed after rhIL-1ra injection in the control groups.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bamba H., Ota S., Kato A., Matsuzaki F. Nonsteroidal anti-inflammatory drugs may delay the repair of gastric mucosa by suppressing prostaglandin-mediated increase of hepatocyte growth factor production. Biochem Biophys Res Commun. 1998 Apr 17;245(2):567–571. doi: 10.1006/bbrc.1998.8436. [DOI] [PubMed] [Google Scholar]
- Beales I. L., Calam J. Interleukin 1 beta and tumour necrosis factor alpha inhibit acid secretion in cultured rabbit parietal cells by multiple pathways. Gut. 1998 Feb;42(2):227–234. doi: 10.1136/gut.42.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beales I. L., Post L., Calam J., Yamada T., Delvalle J. Tumour necrosis factor alpha stimulates gastrin release from canine and human antral G cells: possible mechanism of the Helicobacter pylori-gastrin link. Eur J Clin Invest. 1996 Jul;26(7):609–611. doi: 10.1046/j.1365-2362.1996.2040517.x. [DOI] [PubMed] [Google Scholar]
- Beales I., Blaser M. J., Srinivasan S., Calam J., Pérez-Pérez G. I., Yamada T., Scheiman J., Post L., Del Valle J. Effect of Helicobacter pylori products and recombinant cytokines on gastrin release from cultured canine G cells. Gastroenterology. 1997 Aug;113(2):465–471. doi: 10.1053/gast.1997.v113.pm9247465. [DOI] [PubMed] [Google Scholar]
- Bensi G., Raugei G., Palla E., Carinci V., Tornese Buonamassa D., Melli M. Human interleukin-1 beta gene. Gene. 1987;52(1):95–101. doi: 10.1016/0378-1119(87)90398-2. [DOI] [PubMed] [Google Scholar]
- Blaser M. J. Helicobacter pylori and the pathogenesis of gastroduodenal inflammation. J Infect Dis. 1990 Apr;161(4):626–633. doi: 10.1093/infdis/161.4.626. [DOI] [PubMed] [Google Scholar]
- Courillon-Mallet A., Launay J. M., Roucayrol A. M., Callebert J., Emond J. P., Tabuteau F., Cattan D. Helicobacter pylori infection: physiopathologic implication of N alpha-methyl histamine. Gastroenterology. 1995 Apr;108(4):959–966. doi: 10.1016/0016-5085(95)90190-6. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A. Interleukin-1 and interleukin-1 antagonism. Blood. 1991 Apr 15;77(8):1627–1652. [PubMed] [Google Scholar]
- Dixon M. F., Genta R. M., Yardley J. H., Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996 Oct;20(10):1161–1181. doi: 10.1097/00000478-199610000-00001. [DOI] [PubMed] [Google Scholar]
- Eisenberg S. P., Brewer M. T., Verderber E., Heimdal P., Brandhuber B. J., Thompson R. C. Interleukin 1 receptor antagonist is a member of the interleukin 1 gene family: evolution of a cytokine control mechanism. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5232–5236. doi: 10.1073/pnas.88.12.5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Omar E. M., Carrington M., Chow W. H., McColl K. E., Bream J. H., Young H. A., Herrera J., Lissowska J., Yuan C. C., Rothman N. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature. 2000 Mar 23;404(6776):398–402. doi: 10.1038/35006081. [DOI] [PubMed] [Google Scholar]
- El-Omar E. M., Oien K., El-Nujumi A., Gillen D., Wirz A., Dahill S., Williams C., Ardill J. E., McColl K. E. Helicobacter pylori infection and chronic gastric acid hyposecretion. Gastroenterology. 1997 Jul;113(1):15–24. doi: 10.1016/s0016-5085(97)70075-1. [DOI] [PubMed] [Google Scholar]
- Fraser A. G., Sawyerr A. M., Hudson M., Smith M., Pounder R. E. Effects of ranitidine 150 mg four times a day on 24-hour intragastric acidity and 24-hour plasma gastrin concentration. Dig Dis Sci. 1994 Jan;39(1):91–96. doi: 10.1007/BF02090066. [DOI] [PubMed] [Google Scholar]
- Furuta T., Baba S., Takashima M., Futami H., Arai H., Kajimura M., Hanai H., Kaneko E. Effect of Helicobacter pylori infection on gastric juice pH. Scand J Gastroenterol. 1998 Apr;33(4):357–363. doi: 10.1080/00365529850170973. [DOI] [PubMed] [Google Scholar]
- Furuta T., Baba S., Takashima M., Shirai N., Xiao F., Futami H., Arai H., Hanai H., Kaneko E. H+/K+-adenosine triphosphatase mRNA in gastric fundic gland mucosa in patients infected with Helicobacter pylori. Scand J Gastroenterol. 1999 Apr;34(4):384–390. doi: 10.1080/003655299750026399. [DOI] [PubMed] [Google Scholar]
- Gutierrez O., Melo M., Segura A. M., Angel A., Genta R. M., Graham D. Y. Cure of Helicobacter pylori infection improves gastric acid secretion in patients with corpus gastritis. Scand J Gastroenterol. 1997 Jul;32(7):664–668. doi: 10.3109/00365529708996515. [DOI] [PubMed] [Google Scholar]
- Haruma K., Mihara M., Okamoto E., Kusunoki H., Hananoki M., Tanaka S., Yoshihara M., Sumii K., Kajiyama G. Eradication of Helicobacter pylori increases gastric acidity in patients with atrophic gastritis of the corpus-evaluation of 24-h pH monitoring. Aliment Pharmacol Ther. 1999 Feb;13(2):155–162. doi: 10.1046/j.1365-2036.1999.00459.x. [DOI] [PubMed] [Google Scholar]
- Hassall E., Dimmick J. E. Unique features of Helicobacter pylori disease in children. Dig Dis Sci. 1991 Apr;36(4):417–423. doi: 10.1007/BF01298868. [DOI] [PubMed] [Google Scholar]
- Hirayama F., Takagi S., Kusuhara H., Iwao E., Yokoyama Y., Ikeda Y. Induction of gastric ulcer and intestinal metaplasia in mongolian gerbils infected with Helicobacter pylori. J Gastroenterol. 1996 Oct;31(5):755–757. doi: 10.1007/BF02347631. [DOI] [PubMed] [Google Scholar]
- Hirayama F., Takagi S., Yokoyama Y., Iwao E., Ikeda Y. Establishment of gastric Helicobacter pylori infection in Mongolian gerbils. J Gastroenterol. 1996 Nov;31 (Suppl 9):24–28. [PubMed] [Google Scholar]
- Hurlimann S., Dür S., Schwab P., Varga L., Mazzucchelli L., Brand R., Halter F. Effects of Helicobacter pylori on gastritis, pentagastrin-stimulated gastric acid secretion, and meal-stimulated plasma gastrin release in the absence of peptic ulcer disease. Am J Gastroenterol. 1998 Aug;93(8):1277–1285. doi: 10.1111/j.1572-0241.1998.409_x.x. [DOI] [PubMed] [Google Scholar]
- Ikeno T., Ota H., Sugiyama A., Ishida K., Katsuyama T., Genta R. M., Kawasaki S. Helicobacter pylori-induced chronic active gastritis, intestinal metaplasia, and gastric ulcer in Mongolian gerbils. Am J Pathol. 1999 Mar;154(3):951–960. doi: 10.1016/S0002-9440(10)65343-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablonowski H., Hengels K. J., Kraemer N., Geis G., Opferkuch W., Strohmeyer G. Effects of Helicobacter pylori on histamine and carbachol stimulated acid secretion by human parietal cells. Gut. 1994 Jun;35(6):755–757. doi: 10.1136/gut.35.6.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnes W. E., Jr, Ohning G. V., Sytnik B., Kim S. W., Walsh J. H. Elevation of meal-stimulated gastrin release in subjects with Helicobacter pylori infection: reversal by low intragastric pH. Rev Infect Dis. 1991 Jul-Aug;13 (Suppl 8):S665–S670. doi: 10.1093/clinids/13.supplement_8.s665. [DOI] [PubMed] [Google Scholar]
- Keto Y., Takahashi S., Okabe S. Healing of Helicobacter pylori-induced gastric ulcers in Mongolian gerbils: combined treatment with omeprazole and clarithromycin. Dig Dis Sci. 1999 Feb;44(2):257–265. doi: 10.1023/a:1026685929992. [DOI] [PubMed] [Google Scholar]
- Kondo S., Shinomura Y., Kanayama S., Kawabata S., Miyazaki Y., Imamura I., Fukui H., Matsuzawa Y. Interleukin-1 beta inhibits gastric histamine secretion and synthesis in the rat. Am J Physiol. 1994 Dec;267(6 Pt 1):G966–G971. doi: 10.1152/ajpgi.1994.267.6.G966. [DOI] [PubMed] [Google Scholar]
- Konturek J. W., Gillessen A., Konturek S. J., Domschke W. Eradication of Helicobacter pylori restores the inhibitory effect of cholecystokinin on postprandial gastrin release in duodenal ulcer patients. Gut. 1995 Oct;37(4):482–487. doi: 10.1136/gut.37.4.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenberger L. M., Dial E. J., Romero J. J., Lechago J., Jarboe L. A., Wolfe M. M. Role of luminal ammonia in the development of gastropathy and hypergastrinemia in the rat. Gastroenterology. 1995 Feb;108(2):320–329. doi: 10.1016/0016-5085(95)90056-x. [DOI] [PubMed] [Google Scholar]
- March C. J., Mosley B., Larsen A., Cerretti D. P., Braedt G., Price V., Gillis S., Henney C. S., Kronheim S. R., Grabstein K. Cloning, sequence and expression of two distinct human interleukin-1 complementary DNAs. Nature. 1985 Jun 20;315(6021):641–647. doi: 10.1038/315641a0. [DOI] [PubMed] [Google Scholar]
- Marshall B. J., Goodwin C. S., Warren J. R., Murray R., Blincow E. D., Blackbourn S. J., Phillips M., Waters T. E., Sanderson C. R. Prospective double-blind trial of duodenal ulcer relapse after eradication of Campylobacter pylori. Lancet. 1988 Dec 24;2(8626-8627):1437–1442. doi: 10.1016/s0140-6736(88)90929-4. [DOI] [PubMed] [Google Scholar]
- Marshall B. J., Warren J. R. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984 Jun 16;1(8390):1311–1315. doi: 10.1016/s0140-6736(84)91816-6. [DOI] [PubMed] [Google Scholar]
- Matsuyama S., Koide Y., Yoshida T. O. HLA class II molecule-mediated signal transduction mechanism responsible for the expression of interleukin-1 beta and tumor necrosis factor-alpha genes induced by a staphylococcal superantigen. Eur J Immunol. 1993 Dec;23(12):3194–3202. doi: 10.1002/eji.1830231223. [DOI] [PubMed] [Google Scholar]
- Moss S. F., Calam J. Acid secretion and sensitivity to gastrin in patients with duodenal ulcer: effect of eradication of Helicobacter pylori. Gut. 1993 Jul;34(7):888–892. doi: 10.1136/gut.34.7.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noach L. A., Bosma N. B., Jansen J., Hoek F. J., van Deventer S. J., Tytgat G. N. Mucosal tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-8 production in patients with Helicobacter pylori infection. Scand J Gastroenterol. 1994 May;29(5):425–429. doi: 10.3109/00365529409096833. [DOI] [PubMed] [Google Scholar]
- Nompleggi D. J., Beinborn M., Roy A., Wolfe M. M. The effect of recombinant cytokines on [14C]-aminopyrine accumulation by isolated canine parietal cells. J Pharmacol Exp Ther. 1994 Aug;270(2):440–445. [PubMed] [Google Scholar]
- Ohtsuki T., Ruetzler C. A., Tasaki K., Hallenbeck J. M. Interleukin-1 mediates induction of tolerance to global ischemia in gerbil hippocampal CA1 neurons. J Cereb Blood Flow Metab. 1996 Nov;16(6):1137–1142. doi: 10.1097/00004647-199611000-00007. [DOI] [PubMed] [Google Scholar]
- Piccirilli J. A., Krauch T., Moroney S. E., Benner S. A. Enzymatic incorporation of a new base pair into DNA and RNA extends the genetic alphabet. Nature. 1990 Jan 4;343(6253):33–37. doi: 10.1038/343033a0. [DOI] [PubMed] [Google Scholar]
- Prinz C., Neumayer N., Mahr S., Classen M., Schepp W. Functional impairment of rat enterochromaffin-like cells by interleukin 1 beta. Gastroenterology. 1997 Feb;112(2):364–375. doi: 10.1053/gast.1997.v112.pm9024290. [DOI] [PubMed] [Google Scholar]
- Robert A. Cytoprotection by prostaglandins. Gastroenterology. 1979 Oct;77(4 Pt 1):761–767. [PubMed] [Google Scholar]
- Robert A., Olafsson A. S., Lancaster C., Zhang W. R. Interleukin-1 is cytoprotective, antisecretory, stimulates PGE2 synthesis by the stomach, and retards gastric emptying. Life Sci. 1991;48(2):123–134. doi: 10.1016/0024-3205(91)90405-z. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telford J. L., Macchia G., Massone A., Carinci V., Palla E., Melli M. The murine interleukin 1 beta gene: structure and evolution. Nucleic Acids Res. 1986 Dec 22;14(24):9955–9963. doi: 10.1093/nar/14.24.9955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace J. L., Cucala M., Mugridge K., Parente L. Secretagogue-specific effects of interleukin-1 on gastric acid secretion. Am J Physiol. 1991 Oct;261(4 Pt 1):G559–G564. doi: 10.1152/ajpgi.1991.261.4.G559. [DOI] [PubMed] [Google Scholar]
- Watanabe T., Tada M., Nagai H., Sasaki S., Nakao M. Helicobacter pylori infection induces gastric cancer in mongolian gerbils. Gastroenterology. 1998 Sep;115(3):642–648. doi: 10.1016/s0016-5085(98)70143-x. [DOI] [PubMed] [Google Scholar]
- Weigert N., Schaffer K., Schusdziarra V., Classen M., Schepp W. Gastrin secretion from primary cultures of rabbit antral G cells: stimulation by inflammatory cytokines. Gastroenterology. 1996 Jan;110(1):147–154. doi: 10.1053/gast.1996.v110.pm8536851. [DOI] [PubMed] [Google Scholar]
- Yamaoka Y., Kita M., Kodama T., Sawai N., Kashima K., Imanishi J. Induction of various cytokines and development of severe mucosal inflammation by cagA gene positive Helicobacter pylori strains. Gut. 1997 Oct;41(4):442–451. doi: 10.1136/gut.41.4.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasunaga Y., Shinomura Y., Kanayama S., Higashimoto Y., Yabu M., Miyazaki Y., Kondo S., Murayama Y., Nishibayashi H., Kitamura S. Increased production of interleukin 1 beta and hepatocyte growth factor may contribute to foveolar hyperplasia in enlarged fold gastritis. Gut. 1996 Dec;39(6):787–794. doi: 10.1136/gut.39.6.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el-Omar E., Penman I., Dorrian C. A., Ardill J. E., McColl K. E. Eradicating Helicobacter pylori infection lowers gastrin mediated acid secretion by two thirds in patients with duodenal ulcer. Gut. 1993 Aug;34(8):1060–1065. doi: 10.1136/gut.34.8.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]