Abstract
BACKGROUND—Most patients with alcohol induced cirrhosis (AC) and chronic endotoxinaemia are not suffering from clinically evident systemic inflammatory reactions. This may be due to altered gene expression of cytokines, possibly related to endotoxin (for example, tolerance and sensitisation). Interleukin 18 (IL-18; interleukin γ inducing factor) modulates local cytokine production in response to endotoxin (lipopolysaccharide (LPS)). AIM—To investigate the systemic immune response of patients with AC and to see if unstimulated peripheral blood mononuclear cells (PBMC) from patients with AC are activated and contribute to gene expression of IL-18. METHODS—Plasma levels of endotoxin (LPS) and serum levels of IL-18 were measured by enzyme linked immunoassay and the amoebocyte lysate test in 74 abstinent patients with different stages of AC (Child-Pugh stage A, n=18; B, n=22; C, n=34) and compared with healthy controls (n=43). Gene expression of IL-18 was assessed by semiquantitative reverse transcription-polymerase chain reaction in freshly isolated unstimulated PBMC of a subgroup of 14 patients with AC compared with five healthy controls. RESULTS—Gene expression of IL-18 specific mRNA in unstimulated PBMC was significantly enhanced in patients with advanced AC (Child-Pugh stage C) and correlated with plasma LPS and serum CD14 levels (Spearman rank correlation factors r=0.76 and r=0.72). Serum concentrations of IL-18 were also elevated compared with healthy controls (p<0.001) but correlation with serum levels of CD14 and plasma levels of LPS was much weaker compared with mRNA data (Spearman rank correlation factors r=0.47 and r=0.26). CONCLUSIONS—Our in vivo data suggest a presensitisation of "unstimulated" PBMC in the circulation of patients with AC by endotoxin. The term "unstimulated" may be inadequate in patients with AC. Further investigations are needed to define the exact mechanisms and localisation of sensitisation of PBMC in vivo. Keywords: liver cirrhosis; peripheral blood mononuclear cells; interleukin 18; lipopolysaccharide
Full Text
The Full Text of this article is available as a PDF (184.6 KB).
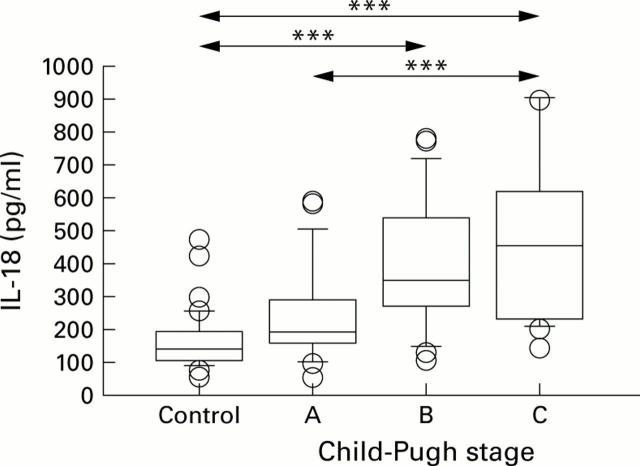
Figure 1 .
Interleukin 18 (IL-18) serum levels (ELISA) were significantly increased in patients with alcohol induced cirrhosis with Child-Pugh stage C disease (n=34; median 456.15 pg/ml; range 163.1-1643.0) compared with healthy controls (n=43; median 135.5 pg/ml; range 62.9-476.1) and patients with Child-Pugh stage A (n=18; median 195.25 pg/ml; range 59.8-598.9). Moreover, significant differences were found between healthy controls and Child-Pugh stage B (n=22; median 355.15 pg/ml; range 119.8-792.9). Box plots indicate the median and 95% confidence intervals (CI). Individual data points are mean serum levels above or below the 95% CI. ***p<0.001.
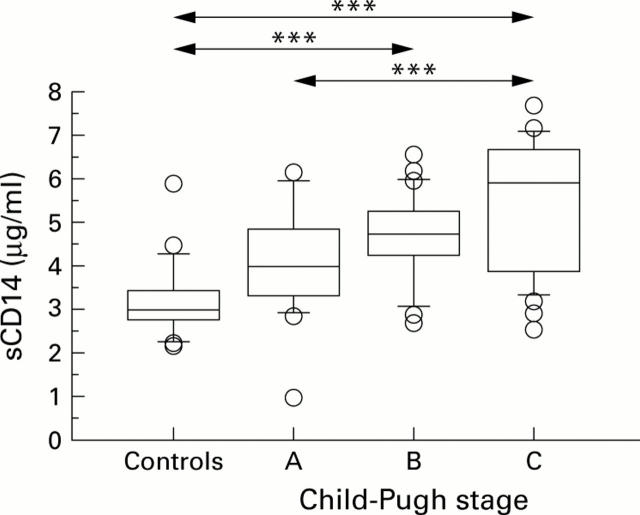
Figure 2 .
CD14 serum levels (ELISA) were significantly increased in patients with alcohol induced cirrhosis with Child-Pugh stage C disease (n=34; median 5.95 µg/ml; range 2.62-7.71) compared with healthy controls (n=43; median 2.98 µg/ml; range 2.19-5.9) and patients with Child-Pugh stage A (n=18; median 4.0 µg/ml; range 1-6.18). Moreover, significant differences were found between healthy controls and Child-Pugh stage B (n=22; median 4.8 µg/ml; range 2.86-6.54). Box plots show median and 95% confidence intervals (CI). Individual data points are mean serum levels above or below the 95% CI. ***p<0.001.
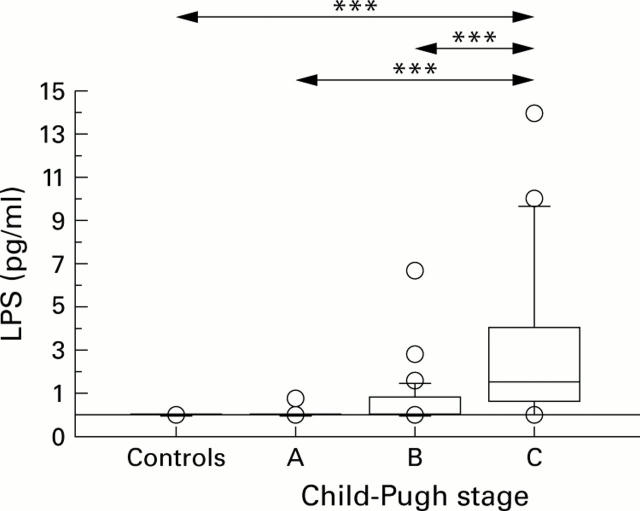
Figure 3 .
Lipopolysaccharide (LPS) plasma levels were significantly increased in patients with alcohol induced cirrhosis with Child-Pugh stage C disease (n=34; median 1.4 pg/ml; range 0-63) compared with healthy controls (n=43; median 0 pg/ml; range 0-0) and patients with Child-Pugh stage A (n=18; median 0 pg/ml; range 0-0.7). Moreover, significant differences were found between healthy controls and Child-Pugh stage B (n=22; median 0 pg/ml; range 0-6.7). Box plots show median and 95% confidence intervals (CI). Individual data points are mean serum levels above or below the 95% CI. ***p<0.001.
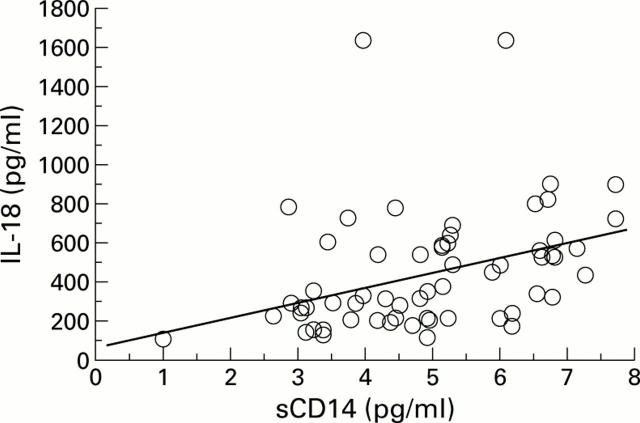
Figure 4 .
Spearman rank analysis reveals a significant correlation between serum levels of interleukin 18 (IL-18) and CD14 (r= 0.47, p<0.001).
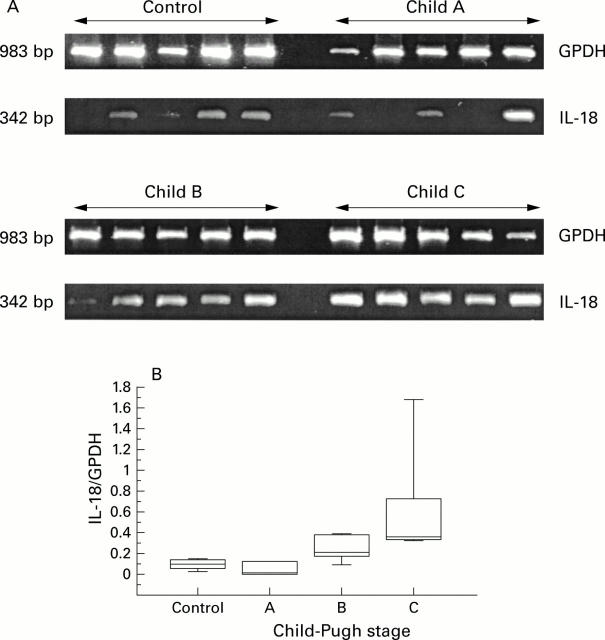
Figure 5 .
(A) mRNA expression of human interleukin 18 (IL-18) in unstimulated freshly isolated peripheral blood mononuclear cells of patients with alcohol induced cirrhosis (Child's stage A-C; n=14) compared with healthy controls (n=5). One patient with Child-Pugh stage A was retrospectively excluded as this patient did not fulfill the inclusion criteria (for example, a single drink/alcohol intake shortly before liver biopsy and venous puncture; histologically no alcoholic hepatitis; no blood parameters of chronic alcohol consumption either at the time of venous puncture or at previous examinations in the outpatient department for more than two years). GPDH, glyceraldehyde 3-phosphate dehydrogenase. (B) Semiquantitative reverse transcription-polymerase chain reaction (RT-PCR) (ratio of mRNA of IL-18/GPDH) revealed that significantly more RT mRNA was expressed in Child's stages B/C than in stage A. Median (range) for Child's stage A, 0.02 (0.01-0.15); for Child's stage B, 0.21 (0.09-0.38); and for Child's stage C, 0.39 (0.32-1.69). Box plots show median and 95% confidence intervals (CI). Individual data points are mean serum levels above or below the 95% CI. *p<0.05.
Figure 6 .
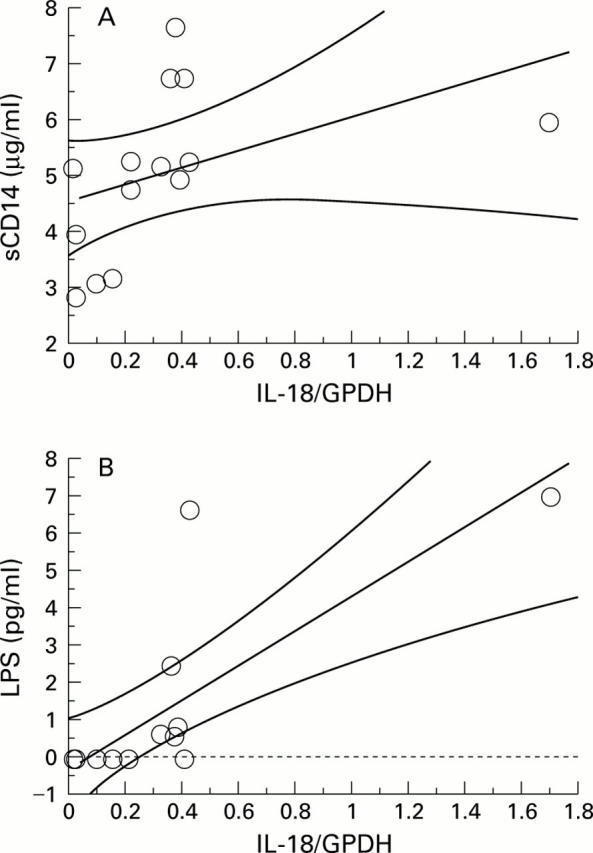
(A) Spearman rank analysis demonstrated a significant correlation between mRNA expression of interleukin 18 (IL-18) in unstimulated peripheral blood mononuclear cells of patients with alcohol induced cirrhosis and sCD14 (r= 0.72; p < 0.01). (B) Spearman rank analysis demonstrated a significant correlation between mRNA expression of IL-18 and lipopolysaccharide (LPS) (r=0.76; p<0.01). GPDH, glyceraldehyde 3-phosphate dehydrogenase.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bohn E., Sing A., Zumbihl R., Bielfeldt C., Okamura H., Kurimoto M., Heesemann J., Autenrieth I. B. IL-18 (IFN-gamma-inducing factor) regulates early cytokine production in, and promotes resolution of, bacterial infection in mice. J Immunol. 1998 Jan 1;160(1):299–307. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Daemen M. A., van't Veer C., Wolfs T. G., Buurman W. A. Ischemia/reperfusion-induced IFN-gamma up-regulation: involvement of IL-12 and IL-18. J Immunol. 1999 May 1;162(9):5506–5510. [PubMed] [Google Scholar]
- Devière J., Vaerman J. P., Content J., Denys C., Schandene L., Vandenbussche P., Sibille Y., Dupont E. IgA triggers tumor necrosis factor alpha secretion by monocytes: a study in normal subjects and patients with alcoholic cirrhosis. Hepatology. 1991 Apr;13(4):670–675. [PubMed] [Google Scholar]
- Diez-Ruiz A., Tilz G. P., Gutierrez-Gea F., Gil-Extremera B., Murr C., Wachter H., Fuchs D. Neopterin and soluble tumor necrosis factor receptor type I in alcohol-induced cirrhosis. Hepatology. 1995 Apr;21(4):976–978. doi: 10.1002/hep.1840210414. [DOI] [PubMed] [Google Scholar]
- Ditter B., Becker K. P., Urbaschek R., Urbaschek B. Quantitativer Endotoxin-Nachweis. Automatisierter, kinetischer Limulus-Amöbozyten-Lysat-Mikrotiter-Test mit Messung probenabhängiger Interferenzen. Arzneimittelforschung. 1983;33(5):681–687. [PubMed] [Google Scholar]
- Enomoto N., Ikejima K., Bradford B., Rivera C., Kono H., Brenner D. A., Thurman R. G. Alcohol causes both tolerance and sensitization of rat Kupffer cells via mechanisms dependent on endotoxin. Gastroenterology. 1998 Aug;115(2):443–451. doi: 10.1016/s0016-5085(98)70211-2. [DOI] [PubMed] [Google Scholar]
- Fantuzzi G., Puren A. J., Harding M. W., Livingston D. J., Dinarello C. A. Interleukin-18 regulation of interferon gamma production and cell proliferation as shown in interleukin-1beta-converting enzyme (caspase-1)-deficient mice. Blood. 1998 Mar 15;91(6):2118–2125. [PubMed] [Google Scholar]
- Fukui H., Brauner B., Bode J. C., Bode C. Plasma endotoxin concentrations in patients with alcoholic and non-alcoholic liver disease: reevaluation with an improved chromogenic assay. J Hepatol. 1991 Mar;12(2):162–169. doi: 10.1016/0168-8278(91)90933-3. [DOI] [PubMed] [Google Scholar]
- Hanck C., Glatzel M., Singer M. V., Rossol S. Gene expression of TNF-receptors in peripheral blood mononuclear cells of patients with alcoholic cirrhosis. J Hepatol. 2000 Jan;32(1):51–57. doi: 10.1016/s0168-8278(00)80189-0. [DOI] [PubMed] [Google Scholar]
- Hanck C., Rossol S., Böcker U., Tokus M., Singer M. V. Presence of plasma endotoxin is correlated with tumour necrosis factor receptor levels and disease activity in alcoholic cirrhosis. Alcohol Alcohol. 1998 Nov-Dec;33(6):606–608. doi: 10.1093/alcalc/33.6.606. [DOI] [PubMed] [Google Scholar]
- Kim Y. M., Kang H. S., Paik S. G., Pyun K. H., Anderson K. L., Torbett B. E., Choi I. Roles of IFN consensus sequence binding protein and PU.1 in regulating IL-18 gene expression. J Immunol. 1999 Aug 15;163(4):2000–2007. [PubMed] [Google Scholar]
- Klein S. A., Ottmann O. G., Ballas K., Dobmeyer T. S., Pape M., Weidmann E., Hoelzer D., Kalina U. Quantification of human interleukin 18 mRNA expression by competitive reverse transcriptase polymerase chain reaction. Cytokine. 1999 Jun;11(6):451–458. doi: 10.1006/cyto.1998.0424. [DOI] [PubMed] [Google Scholar]
- Knolle P. A., Germann T., Treichel U., Uhrig A., Schmitt E., Hegenbarth S., Lohse A. W., Gerken G. Endotoxin down-regulates T cell activation by antigen-presenting liver sinusoidal endothelial cells. J Immunol. 1999 Feb 1;162(3):1401–1407. [PubMed] [Google Scholar]
- Laso F. J., Lapeña P., Madruga J. I., San Miguel J. F., Orfao A., Iglesias M. C., Alvarez-Mon M. Alterations in tumor necrosis factor-alpha, interferon-gamma, and interleukin-6 production by natural killer cell-enriched peripheral blood mononuclear cells in chronic alcoholism: relationship with liver disease and ethanol intake. Alcohol Clin Exp Res. 1997 Oct;21(7):1226–1231. [PubMed] [Google Scholar]
- Luna-Casado L., Diez-Ruiz A., Gutierrez-Gea F., Santos-Perez J. L., Rico-Irles J., Wachter H., Fuchs D. Increased peripheral mononuclear cells expression of adhesion molecules in alcoholic cirrhosis: its relation to immune activation. J Hepatol. 1997 Sep;27(3):477–483. doi: 10.1016/s0168-8278(97)80351-0. [DOI] [PubMed] [Google Scholar]
- Maddrey W. C., Boitnott J. K., Bedine M. S., Weber F. L., Jr, Mezey E., White R. I., Jr Corticosteroid therapy of alcoholic hepatitis. Gastroenterology. 1978 Aug;75(2):193–199. [PubMed] [Google Scholar]
- Mandrekar P., Catalano D., Szabo G. Alcohol-induced regulation of nuclear regulatory factor-kappa beta in human monocytes. Alcohol Clin Exp Res. 1997 Sep;21(6):988–994. [PubMed] [Google Scholar]
- Marshall J. D., Aste-Amézaga M., Chehimi S. S., Murphy M., Olsen H., Trinchieri G. Regulation of human IL-18 mRNA expression. Clin Immunol. 1999 Jan;90(1):15–21. doi: 10.1006/clim.1998.4633. [DOI] [PubMed] [Google Scholar]
- Mastroeni P., Clare S., Khan S., Harrison J. A., Hormaeche C. E., Okamura H., Kurimoto M., Dougan G. Interleukin 18 contributes to host resistance and gamma interferon production in mice infected with virulent Salmonella typhimurium. Infect Immun. 1999 Feb;67(2):478–483. doi: 10.1128/iai.67.2.478-483.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogensen C. E. The glomerular permeability determined by dextran clearance using Sephadex gel filtration. Scand J Clin Lab Invest. 1968;21(1):77–82. doi: 10.3109/00365516809076979. [DOI] [PubMed] [Google Scholar]
- Monteleone G., Trapasso F., Parrello T., Biancone L., Stella A., Iuliano R., Luzza F., Fusco A., Pallone F. Bioactive IL-18 expression is up-regulated in Crohn's disease. J Immunol. 1999 Jul 1;163(1):143–147. [PubMed] [Google Scholar]
- Naveau S., Emilie D., Balian A., Grangeot-Keros L., Borotto E., Portier A., Giraud V., Capron F., Galanaud P., Chaput J. C. Plasma levels of soluble tumor necrosis factor receptors p55 and p75 in patients with alcoholic liver disease of increasing severity. J Hepatol. 1998 May;28(5):778–784. doi: 10.1016/s0168-8278(98)80227-4. [DOI] [PubMed] [Google Scholar]
- Okamura H., Tsutsi H., Komatsu T., Yutsudo M., Hakura A., Tanimoto T., Torigoe K., Okura T., Nukada Y., Hattori K. Cloning of a new cytokine that induces IFN-gamma production by T cells. Nature. 1995 Nov 2;378(6552):88–91. doi: 10.1038/378088a0. [DOI] [PubMed] [Google Scholar]
- Pasqualetti P., Festuccia V., MacCarone C., Di Lauro G., Casale R. Valore diagnostico della gamma-glutamiltranspeptidasi e del volume corpuscolare medio per l'etiologia alcolica in corso di epatopatia cronica. Minerva Med. 1995 Oct;86(10):395–402. [PubMed] [Google Scholar]
- Pizarro T. T., Michie M. H., Bentz M., Woraratanadharm J., Smith M. F., Jr, Foley E., Moskaluk C. A., Bickston S. J., Cominelli F. IL-18, a novel immunoregulatory cytokine, is up-regulated in Crohn's disease: expression and localization in intestinal mucosal cells. J Immunol. 1999 Jun 1;162(11):6829–6835. [PubMed] [Google Scholar]
- Pugh R. N., Murray-Lyon I. M., Dawson J. L., Pietroni M. C., Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973 Aug;60(8):646–649. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- Puren A. J., Fantuzzi G., Dinarello C. A. Gene expression, synthesis, and secretion of interleukin 18 and interleukin 1beta are differentially regulated in human blood mononuclear cells and mouse spleen cells. Proc Natl Acad Sci U S A. 1999 Mar 2;96(5):2256–2261. doi: 10.1073/pnas.96.5.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puren A. J., Fantuzzi G., Gu Y., Su M. S., Dinarello C. A. Interleukin-18 (IFNgamma-inducing factor) induces IL-8 and IL-1beta via TNFalpha production from non-CD14+ human blood mononuclear cells. J Clin Invest. 1998 Feb 1;101(3):711–721. doi: 10.1172/JCI1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rietschel E. T., Kirikae T., Schade F. U., Mamat U., Schmidt G., Loppnow H., Ulmer A. J., Zähringer U., Seydel U., Di Padova F. Bacterial endotoxin: molecular relationships of structure to activity and function. FASEB J. 1994 Feb;8(2):217–225. doi: 10.1096/fasebj.8.2.8119492. [DOI] [PubMed] [Google Scholar]
- Sakao Y., Takeda K., Tsutsui H., Kaisho T., Nomura F., Okamura H., Nakanishi K., Akira S. IL-18-deficient mice are resistant to endotoxin-induced liver injury but highly susceptible to endotoxin shock. Int Immunol. 1999 Mar;11(3):471–480. doi: 10.1093/intimm/11.3.471. [DOI] [PubMed] [Google Scholar]
- Schletter J., Heine H., Ulmer A. J., Rietschel E. T. Molecular mechanisms of endotoxin activity. Arch Microbiol. 1995 Dec;164(6):383–389. doi: 10.1007/BF02529735. [DOI] [PubMed] [Google Scholar]
- Schäfer C., Greiner B., Landig J., Feil E., Schütz E. T., Bode J. C., Bode C. Decreased endotoxin-binding capacity of whole blood in patients with alcoholic liver disease. J Hepatol. 1997 Mar;26(3):567–573. doi: 10.1016/s0168-8278(97)80422-9. [DOI] [PubMed] [Google Scholar]
- Schäfer C., Schips I., Landig J., Bode J. C., Bode C. Tumor-necrosis-factor and interleukin-6 response of peripheral blood monocytes to low concentrations of lipopolysaccharide in patients with alcoholic liver disease. Z Gastroenterol. 1995 Sep;33(9):503–508. [PubMed] [Google Scholar]
- Stoll S., Jonuleit H., Schmitt E., Müller G., Yamauchi H., Kurimoto M., Knop J., Enk A. H. Production of functional IL-18 by different subtypes of murine and human dendritic cells (DC): DC-derived IL-18 enhances IL-12-dependent Th1 development. Eur J Immunol. 1998 Oct;28(10):3231–3239. doi: 10.1002/(SICI)1521-4141(199810)28:10<3231::AID-IMMU3231>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Taniguchi M., Nagaoka K., Kunikata T., Kayano T., Yamauchi H., Nakamura S., Ikeda M., Orita K., Kurimoto M. Characterization of anti-human interleukin-18 (IL-18)/interferon-gamma-inducing factor (IGIF) monoclonal antibodies and their application in the measurement of human IL-18 by ELISA. J Immunol Methods. 1997 Aug 7;206(1-2):107–113. doi: 10.1016/s0022-1759(97)00094-x. [DOI] [PubMed] [Google Scholar]
- Tilg H., Vogel W., Wiedermann C. J., Shapiro L., Herold M., Judmaier G., Dinarello C. A. Circulating interleukin-1 and tumor necrosis factor antagonists in liver disease. Hepatology. 1993 Nov;18(5):1132–1138. [PubMed] [Google Scholar]
- Tsuji H., Mukaida N., Harada A., Kaneko S., Matsushita E., Nakanuma Y., Tsutsui H., Okamura H., Nakanishi K., Tagawa Y. Alleviation of lipopolysaccharide-induced acute liver injury in Propionibacterium acnes-primed IFN-gamma-deficient mice by a concomitant reduction of TNF-alpha, IL-12, and IL-18 production. J Immunol. 1999 Jan 15;162(2):1049–1055. [PubMed] [Google Scholar]
- Ushio S., Namba M., Okura T., Hattori K., Nukada Y., Akita K., Tanabe F., Konishi K., Micallef M., Fujii M. Cloning of the cDNA for human IFN-gamma-inducing factor, expression in Escherichia coli, and studies on the biologic activities of the protein. J Immunol. 1996 Jun 1;156(11):4274–4279. [PubMed] [Google Scholar]
- Watson R. R., Mohs M. E., Eskelson C., Sampliner R. E., Hartmann B. Identification of alcohol abuse and alcoholism with biological parameters. Alcohol Clin Exp Res. 1986 Aug;10(4):364–385. doi: 10.1111/j.1530-0277.1986.tb05108.x. [DOI] [PubMed] [Google Scholar]
- Yoshida M., Roth R. I., Levin J. The effect of cell-free hemoglobin on intravascular clearance and cellular, plasma, and organ distribution of bacterial endotoxin in rabbits. J Lab Clin Med. 1995 Aug;126(2):151–160. [PubMed] [Google Scholar]
- van Deventer S. J., Buller H. R., ten Cate J. W., Sturk A., Pauw W. Endotoxaemia: an early predictor of septicaemia in febrile patients. Lancet. 1988 Mar 19;1(8586):605–609. doi: 10.1016/s0140-6736(88)91412-2. [DOI] [PubMed] [Google Scholar]