Abstract
BACKGROUND—Intestinal metaplasia is considered a risk factor for the development of gastric adenocarcinomas of the intestinal type and is found in approximately 20% of gastric biopsies. Conventional histology only detects advanced stages of intestinal metaplasia. AIMS—To study expression of the enterocyte specific adhesion molecule liver-intestinal (LI)-cadherin in intestinal metaplasia as well as in gastric cancer, and to evaluate its use as a diagnostic marker molecule. PATIENTS—Gastric biopsies (n=77) from 30 consecutive patients (n=30; aged 28-90 years) as well as surgically resected tissue samples (n=24) of all types of gastric carcinomas were analysed. METHODS—Single and double label immunofluorescence detection on cryosections of gastric biopsies; alkaline phosphatase antialkaline phosphatase method on paraffin embedded carcinoma tissue sections. RESULTS—Of 77 biopsies (from 30 patients), 12 (from 10 patients) stained positive for LI-cadherin. LI-cadherin staining correlated with the presence of intestinal metaplasia. Conventional histological diagnosis however failed to detect subtle gastric intestinal metaplasia (three of 10 patients). In contrast, only LI-cadherin and villin were positive in these cases whereas sucrase-isomaltase also failed to detect intestinal metaplasia in four of 10 patients. Well differentiated gastric carcinomas showed intense staining for LI-cadherin while undifferentiated carcinomas showed only weak diffuse cytoplasmic staining. CONCLUSIONS—To detect early metaplastic changes in the gastric mucosa, LI-cadherin has a sensitivity superior to sucrase-isomaltase and conventional histology and comparable with that of villin. Its specificity exceeds that of villin. Thus LI-cadherin represents a new, reliable, and powerful marker molecule for early detection of gastric intestinal metaplasia and well differentiated adenocarcinomas. Keywords: stomach; intestinal metaplasia; cadherins; carcinogenesis
Full Text
The Full Text of this article is available as a PDF (356.4 KB).
Figure 1 .
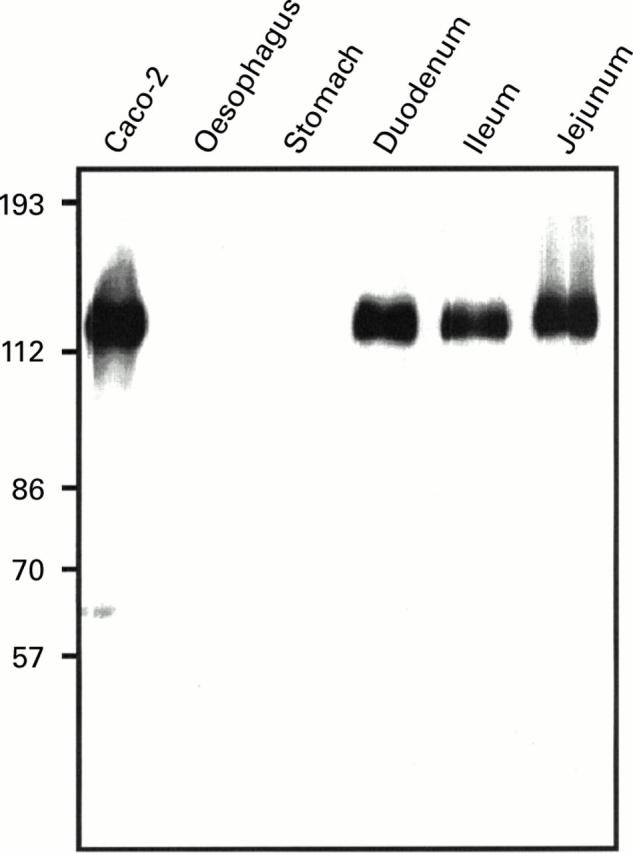
Western blot analysis of LI-cadherin expression in the upper gastrointestinal tract. Protein extracts of biopsies (oesophagus, stomach, duodenum, ileum, and jejunum) and of the human colon carcinoma cell line Caco-2 were separated by sodium dodecyl sulphate-polyacrylamide gel electrophoresis and immunoblotted with polyclonal antiserum against human LI-cadherin.
Figure 2 .
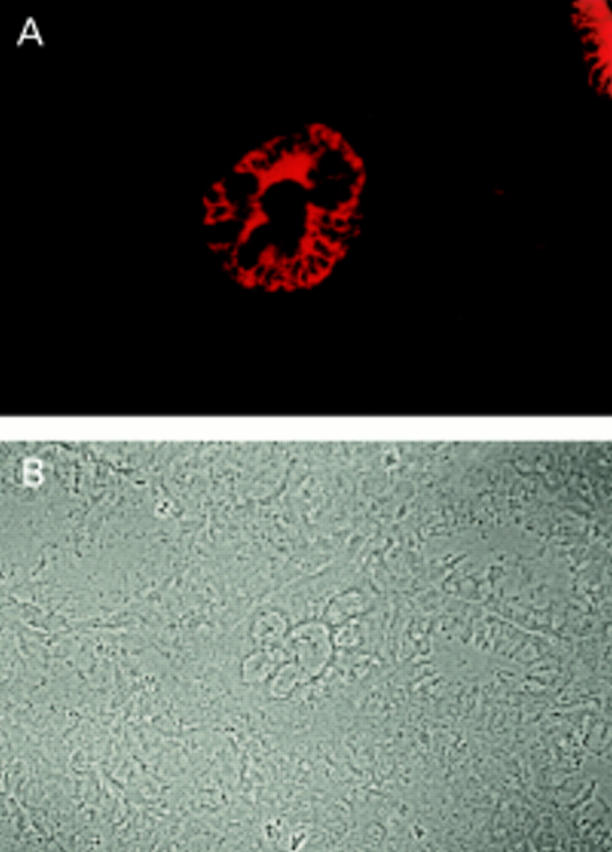
Immunofluorescence microscopy of a gastric biopsy stained with polyclonal antibody against LI-cadherin: (A) fluorescence signal of LI-cadherin, (B) phase contrast. Note the distinct LI-cadherin staining of goblet cells in one gland whereas surrounding glands are negative.
Figure 3 .
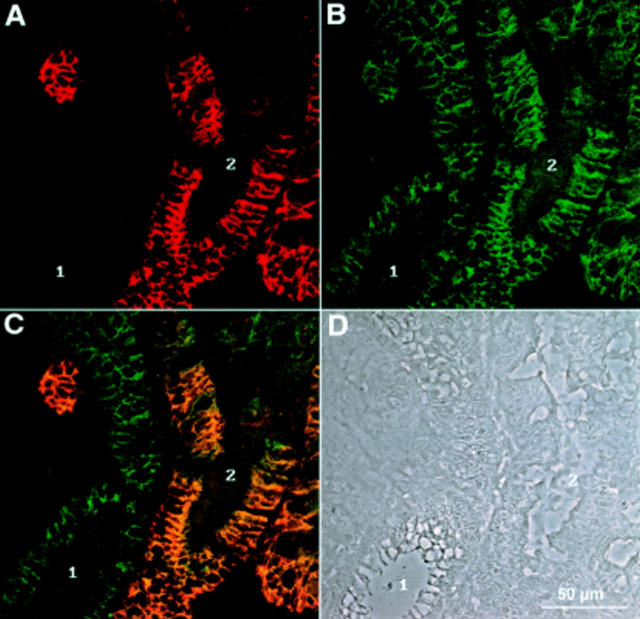
Double immunofluorescence recorded by confocal laser microscopy of a gastric biopsy stained with polyclonal antiserum against LI-cadherin (A) and monoclonal antibody against E-cadherin (B). (C) Combined images of both proteins. The phase contrast image of the same area (D) reveals regions of normal mucosa (1) next to those showing characteristics of intestinal metaplasia (2). Note the high degree of colocalisation in cells expressing both LI-cadherin and E-cadherin.
Figure 4 .
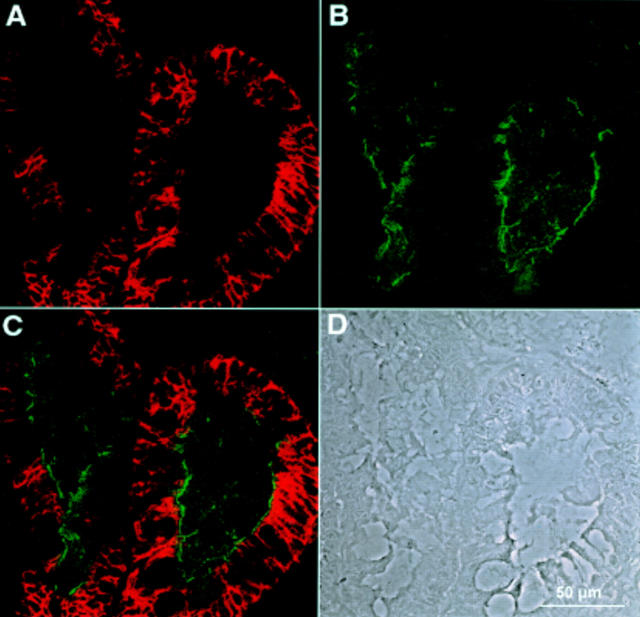
Double immunofluorescence recorded by confocal laser microscopy of a gastric biopsy with polyclonal antiserum against LI-cadherin (A) and monoclonal antibody against sucrase-isomaltase (B). (C) Combined images of both proteins. (D) Phase contrast image of the same area. Whereas LI-cadherin expression is restricted to the basolateral plasma membrane regions, sucrase-isomaltase is exclusively found in the apical regions and does not colocalise with LI-cadherin.
Figure 5 .
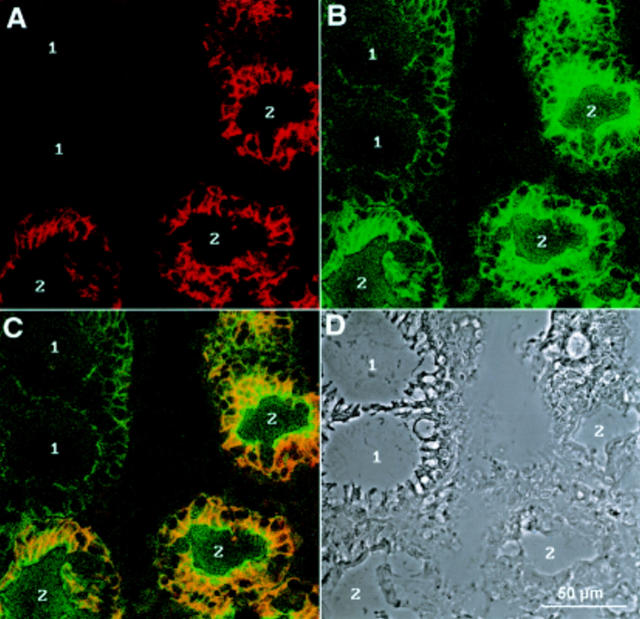
Immunofluorescence recorded by confocal laser microscopy of a gastric biopsy with polyclonal antiserum against LI-cadherin (A) and monoclonal antibody against villin (B). (C) Combined images of both proteins. (D) Phase contrast image of the same area. Whereas LI-cadherin background is very low in non-metaplastic regions (1, A), villin staining results typically in higher background staining (1, B). Connective tissue in between the glands is totally negative for both proteins (centre).
Figure 6 .
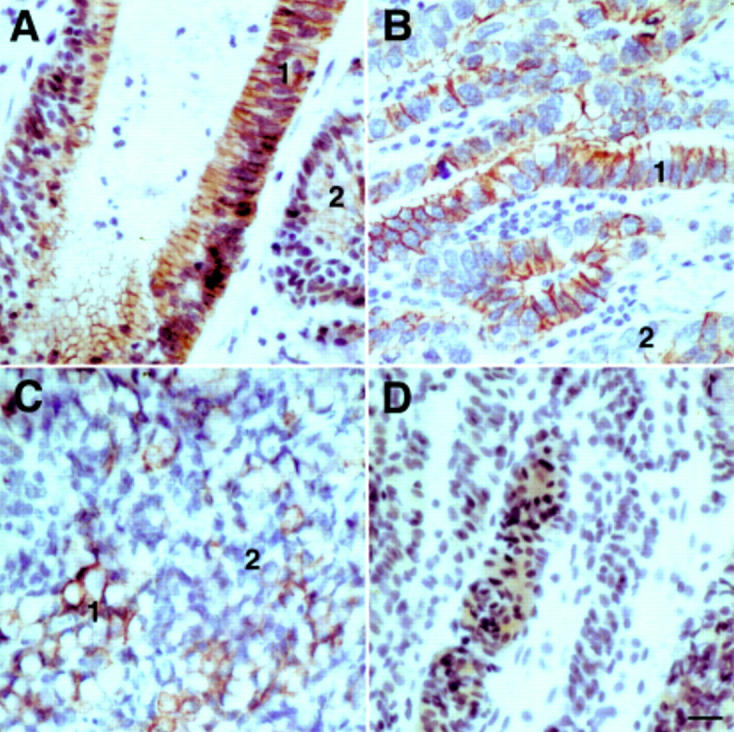
Immunoreactivity of LI-cadherin in gastric carcinomas. Immunohistochemical staining of LI-cadherin in mucinous (A), tubular (B), and signet ring (C) adenocarcinoma, and in undifferentiated carcinoma of the stomach (D). Note the intense staining of the cell-cell contact areas in well differentiated regions (1) of adenocarcinomas contrasted by weaker staining in less differentiated regions (2). The undifferentiated carcinoma (D) exhibits only faint cytoplasmic staining for LI-cadherin.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antonioli D. A. Precursors of gastric carcinoma: a critical review with a brief description of early (curable) gastric cancer. Hum Pathol. 1994 Oct;25(10):994–1005. doi: 10.1016/0046-8177(94)90058-2. [DOI] [PubMed] [Google Scholar]
- Behrens J. The role of cell adhesion molecules in cancer invasion and metastasis. Breast Cancer Res Treat. 1993;24(3):175–184. doi: 10.1007/BF01833258. [DOI] [PubMed] [Google Scholar]
- Berndorff D., Gessner R., Kreft B., Schnoy N., Lajous-Petter A. M., Loch N., Reutter W., Hortsch M., Tauber R. Liver-intestine cadherin: molecular cloning and characterization of a novel Ca(2+)-dependent cell adhesion molecule expressed in liver and intestine. J Cell Biol. 1994 Jun;125(6):1353–1369. doi: 10.1083/jcb.125.6.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birchmeier W., Behrens J. Cadherin expression in carcinomas: role in the formation of cell junctions and the prevention of invasiveness. Biochim Biophys Acta. 1994 May 27;1198(1):11–26. doi: 10.1016/0304-419x(94)90003-5. [DOI] [PubMed] [Google Scholar]
- Borchard F., Heilmann K. L., Hermanek P., Gebbers J. O., Heitz P. U., Stolte M., Pfeifer U., Schaefer H. E., Wiebecke B., Schlake W. Definition und klinische Bedeutung der Dysplasie im Verdauungstrakt. Ergebnisse einer Sitzung der Arbeitsgemeinschaft Gastroenterologische Pathologie der Deutschen Gesellschaft für Pathologie am 25.11.1989 in Kronberg. Pathologe. 1991 Jan;12(1):50–56. [PubMed] [Google Scholar]
- Boring C. C., Squires T. S., Tong T., Montgomery S. Cancer statistics, 1994. CA Cancer J Clin. 1994 Jan-Feb;44(1):7–26. doi: 10.3322/canjclin.44.1.7. [DOI] [PubMed] [Google Scholar]
- Bretscher A., Weber K. Villin: the major microfilament-associated protein of the intestinal microvillus. Proc Natl Acad Sci U S A. 1979 May;76(5):2321–2325. doi: 10.1073/pnas.76.5.2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa P., Haenszel W., Cuello C., Tannenbaum S., Archer M. A model for gastric cancer epidemiology. Lancet. 1975 Jul 12;2(7924):58–60. doi: 10.1016/s0140-6736(75)90498-5. [DOI] [PubMed] [Google Scholar]
- Filipe M. I., Potet F., Bogomoletz W. V., Dawson P. A., Fabiani B., Chauveinc P., Fenzy A., Gazzard B., Goldfain D., Zeegen R. Incomplete sulphomucin-secreting intestinal metaplasia for gastric cancer. Preliminary data from a prospective study from three centres. Gut. 1985 Dec;26(12):1319–1326. doi: 10.1136/gut.26.12.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederich E., Pringault E., Arpin M., Louvard D. From the structure to the function of villin, an actin-binding protein of the brush border. Bioessays. 1990 Sep;12(9):403–408. doi: 10.1002/bies.950120902. [DOI] [PubMed] [Google Scholar]
- Gabbert H. E., Mueller W., Schneiders A., Meier S., Moll R., Birchmeier W., Hommel G. Prognostic value of E-cadherin expression in 413 gastric carcinomas. Int J Cancer. 1996 Jun 21;69(3):184–189. doi: 10.1002/(SICI)1097-0215(19960621)69:3<184::AID-IJC6>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Geiger B., Ayalon O. Cadherins. Annu Rev Cell Biol. 1992;8:307–332. doi: 10.1146/annurev.cb.08.110192.001515. [DOI] [PubMed] [Google Scholar]
- Geiger B., Ginsberg D., Salomon D., Volberg T. The molecular basis for the assembly and modulation of adherens-type junctions. Cell Differ Dev. 1990 Dec 2;32(3):343–353. doi: 10.1016/0922-3371(90)90049-3. [DOI] [PubMed] [Google Scholar]
- Guilford P., Hopkins J., Harraway J., McLeod M., McLeod N., Harawira P., Taite H., Scoular R., Miller A., Reeve A. E. E-cadherin germline mutations in familial gastric cancer. Nature. 1998 Mar 26;392(6674):402–405. doi: 10.1038/32918. [DOI] [PubMed] [Google Scholar]
- Gumbiner B. M. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell. 1996 Feb 9;84(3):345–357. doi: 10.1016/s0092-8674(00)81279-9. [DOI] [PubMed] [Google Scholar]
- Gumbiner B. M. Epithelial morphogenesis. Cell. 1992 May 1;69(3):385–387. doi: 10.1016/0092-8674(92)90440-n. [DOI] [PubMed] [Google Scholar]
- Hauri H. P., Sterchi E. E., Bienz D., Fransen J. A., Marxer A. Expression and intracellular transport of microvillus membrane hydrolases in human intestinal epithelial cells. J Cell Biol. 1985 Sep;101(3):838–851. doi: 10.1083/jcb.101.3.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höfer D., Drenckhahn D. Cytoskeletal markers allowing discrimination between brush cells and other epithelial cells of the gut including enteroendocrine cells. Histochem Cell Biol. 1996 May;105(5):405–412. doi: 10.1007/BF01463662. [DOI] [PubMed] [Google Scholar]
- Iida F., Murata F., Nagata T. Histochemical studies of mucosubstances in metaplastic epithelium of the stomach, with special reference to the development of intestinal metaplasia. Histochemistry. 1978 Jul 12;56(3-4):229–237. doi: 10.1007/BF00495984. [DOI] [PubMed] [Google Scholar]
- Jass J. R., Filipe M. I. A variant of intestinal metaplasia associated with gastric carcinoma: a histochemical study. Histopathology. 1979 May;3(3):191–199. doi: 10.1111/j.1365-2559.1979.tb02996.x. [DOI] [PubMed] [Google Scholar]
- Jawhari A., Jordan S., Poole S., Browne P., Pignatelli M., Farthing M. J. Abnormal immunoreactivity of the E-cadherin-catenin complex in gastric carcinoma: relationship with patient survival. Gastroenterology. 1997 Jan;112(1):46–54. doi: 10.1016/s0016-5085(97)70218-x. [DOI] [PubMed] [Google Scholar]
- Kreft B., Berndorff D., Böttinger A., Finnemann S., Wedlich D., Hortsch M., Tauber R., Gessner R. LI-cadherin-mediated cell-cell adhesion does not require cytoplasmic interactions. J Cell Biol. 1997 Mar 10;136(5):1109–1121. doi: 10.1083/jcb.136.5.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzsonn V., Korsmo H., Olsen W. A. Localization of sucrase-isomaltase in the rat enterocyte. Gastroenterology. 1987 Jan;92(1):98–105. doi: 10.1016/0016-5085(87)90844-4. [DOI] [PubMed] [Google Scholar]
- MORSON B. C. Carcinoma arising from areas of intestinal metaplasia in the gastric mucosa. Br J Cancer. 1955 Sep;9(3):377–385. doi: 10.1038/bjc.1955.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORSON B. C. Intestinal metaplasia of the gastric mucosa. Br J Cancer. 1955 Sep;9(3):365–376. doi: 10.1038/bjc.1955.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsukura N., Suzuki K., Kawachi T., Aoyagi M., Sugimura T., Kitaoka H., Numajiri H., Shirota A., Itabashi M., Hirota T. Distribution of marker enzymes and mucin in intestinal metaplasia in human stomach and relation to complete and incomplete types of intestinal metaplasia to minute gastric carcinomas. J Natl Cancer Inst. 1980 Aug;65(2):231–240. [PubMed] [Google Scholar]
- Mayer B., Johnson J. P., Leitl F., Jauch K. W., Heiss M. M., Schildberg F. W., Birchmeier W., Funke I. E-cadherin expression in primary and metastatic gastric cancer: down-regulation correlates with cellular dedifferentiation and glandular disintegration. Cancer Res. 1993 Apr 1;53(7):1690–1695. [PubMed] [Google Scholar]
- Moll R., Robine S., Dudouet B., Louvard D. Villin: a cytoskeletal protein and a differentiation marker expressed in some human adenocarcinomas. Virchows Arch B Cell Pathol Incl Mol Pathol. 1987;54(3):155–169. doi: 10.1007/BF02899208. [DOI] [PubMed] [Google Scholar]
- Osborn M., Mazzoleni G., Santini D., Marrano D., Martinelli G., Weber K. Villin, intestinal brush border hydrolases and keratin polypeptides in intestinal metaplasia and gastric cancer; an immunohistologic study emphasizing the different degrees of intestinal and gastric differentiation in signet ring cell carcinomas. Virchows Arch A Pathol Anat Histopathol. 1988;413(4):303–312. doi: 10.1007/BF00783022. [DOI] [PubMed] [Google Scholar]
- Shimoyama Y., Hirohashi S. Expression of E- and P-cadherin in gastric carcinomas. Cancer Res. 1991 Apr 15;51(8):2185–2192. [PubMed] [Google Scholar]
- Shino Y., Watanabe A., Yamada Y., Tanase M., Yamada T., Matsuda M., Yamashita J., Tatsumi M., Miwa T., Nakano H. Clinicopathologic evaluation of immunohistochemical E-cadherin expression in human gastric carcinomas. Cancer. 1995 Dec 1;76(11):2193–2201. doi: 10.1002/1097-0142(19951201)76:11<2193::aid-cncr2820761104>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Silva S., Filipe M. I. Intestinal metaplasia and its variants in the gastric mucosa of Portuguese subjects: a comparative analysis of biopsy and gastrectomy material. Hum Pathol. 1986 Oct;17(10):988–995. doi: 10.1016/s0046-8177(86)80082-x. [DOI] [PubMed] [Google Scholar]
- Takeichi M. Morphogenetic roles of classic cadherins. Curr Opin Cell Biol. 1995 Oct;7(5):619–627. doi: 10.1016/0955-0674(95)80102-2. [DOI] [PubMed] [Google Scholar]
- Tosi P., Filipe M. I., Luzi P., Miracco C., Santopietro R., Lio R., Sforza V., Barbini P. Gastric intestinal metaplasia type III cases are classified as low-grade dysplasia on the basis of morphometry. J Pathol. 1993 Jan;169(1):73–78. doi: 10.1002/path.1711690112. [DOI] [PubMed] [Google Scholar]
- Vleminckx K., Vakaet L., Jr, Mareel M., Fiers W., van Roy F. Genetic manipulation of E-cadherin expression by epithelial tumor cells reveals an invasion suppressor role. Cell. 1991 Jul 12;66(1):107–119. doi: 10.1016/0092-8674(91)90143-m. [DOI] [PubMed] [Google Scholar]
- Yasui W., Sano T., Nishimura K., Kitadai Y., Ji Z. Q., Yokozaki H., Ito H., Tahara E. Expression of P-cadherin in gastric carcinomas and its reduction in tumor progression. Int J Cancer. 1993 Apr 22;54(1):49–52. doi: 10.1002/ijc.2910540109. [DOI] [PubMed] [Google Scholar]