Abstract
BACKGROUND—Primary sclerosing cholangitis (PSC) is considered to be a chronic autoimmune disease where infiltrating T lymphocytes have been implicated in the destruction of bile ducts. Altered function of these T cells may reflect abnormalities in the immune response leading to tissue damage. AIM—We investigated the proliferative and functional capacity of freshly isolated liver derived T lymphocytes (LDLs) and natural killer (NK) cells from PSC patients. METHODS—The proliferative responses to common mitogens such as phytohaemagglutinin (PHA), concanavalin A (Con A), and lipopolysaccharide (LPS) were studied, and the cytotoxic function of T lymphocytes was measured using allogeneic target cells. NK (CD56+/16+) cytotoxic function was measured using the two cell lines K562 (NK sensitive) and Raji lymphoma cells (NK resistant). RESULTS—Compared with patients with primary biliary cirrhosis (PBC), autoimmune hepatitis (AIH), and normal controls (without liver disease), in PSC: (1) LDLs contained a low percentage of T cells; (2) there was significantly decreased expression of interleukin (IL)-2 receptor (p<0.001) on activated T cells (HLA-DR+); (3) LDLs but not peripheral blood lymphocytes had significantly impaired proliferative responses to mitogens such as PHA, Con A, and LPS (p< 0.001); (4) no cytotoxic activity of PSC liver T and NK cells was recorded; (5) significantly higher levels of tumour necrosis factor α (TNF-α) and IL-1β but lower levels of IL-2, IL-10, and interferon γ were found in the supernatants of mitogen stimulated LDL cultures (p<0.001); (6) higher percentages of freshly isolated PSC LDLs contained intracytoplasmic TNF-α and IL-1β; and (7) pretreatment of PSC LDLs in vitro with neutralising TNF antibodies significantly enhanced proliferative responses and allowed IL-2 receptor expression following stimulation. In addition, the impaired cytolytic activity of both NK and T cells was partially restored. Impaired proliferative or functional capacity of liver derived T cells was not observed in either PBC or AIH patients. CONCLUSIONS—We suggest that reduced T cell reactivity in liver infiltrating cells obtained from patients with PSC is due to high local production of TNF-α. Our findings indicate that the use of anti-TNF antibodies as an alternative treatment for PSC patients should be evaluated. Keywords: autoimmune liver diseases; biliary epithelial cells; cytokines
Full Text
The Full Text of this article is available as a PDF (209.6 KB).
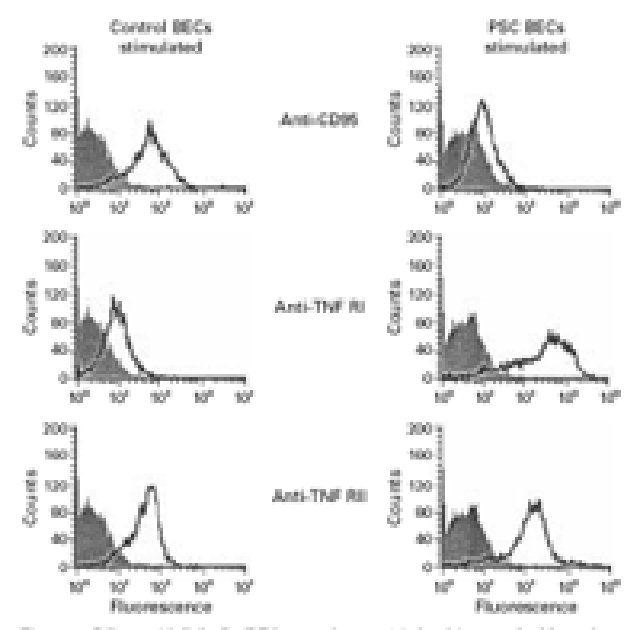
Figure 1 .
Biliary epithelial cells (BECs, second passage) isolated from two healthy and three primary sclerosing cholangitis (PSC) livers were tested for expression of CD95 and the two tumour necrosis factor (TNF) receptors to study the role of BECs as targets for lymphocyte mediated or direct cytokine mediated damage. TNF-α and IL-1 stimulated PSC BECs demonstrated increased expression of the TNF RI and RII receptors but low expression of CD95. Normal BECs expressed CD95 and TNF RII but had lower expression of TNF RI.
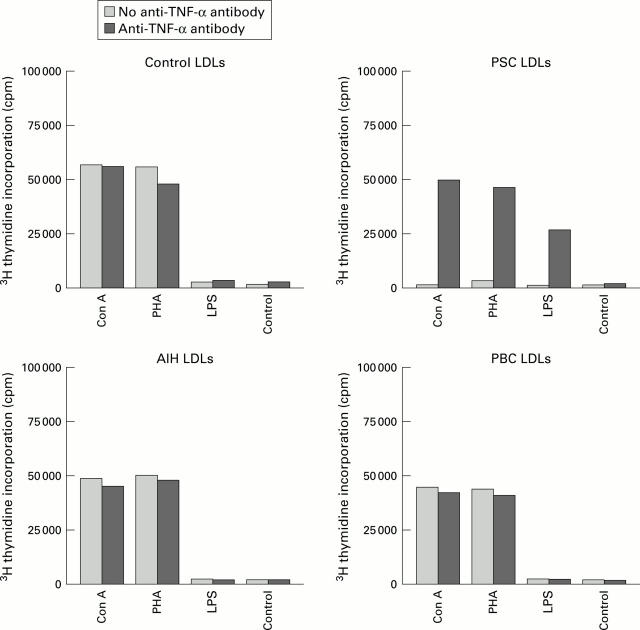
Figure 2 .
In general, liver derived lymphocytes (LDLs) isolated from controls (n=8) on stimulation with mitogens such as concanavalin A (Con A) and phytohaemagglutinin (PHA) for 72 hours showed lower proliferation responses than those observed in peripheral blood lymphocytes from the same on stimulation. No difference in response was observed after pretreatment with anti-tumour necrosis factor (TNF) monoclonal antibodies. Significantly diminished proliferation of LDLs from the seven primary sclerosing cholangitis (PSC) patients was observed on stimulation which was restored to almost normal by pretreatment with anti-TNF monoclonal antibodies (p<0.001). No significant differences in proliferative responses in mitogen stimulated LDLs from primary biliary cirrhosis (PBC) or autoimmune hepatitis (AIH) patients (either before or after anti-TNF monoclonal antibody pretreatment) was observed compared with controls (NS). Data represent mean counts per minute (cpm) of triplicate cultures. LPS, lipopolysaccharide.
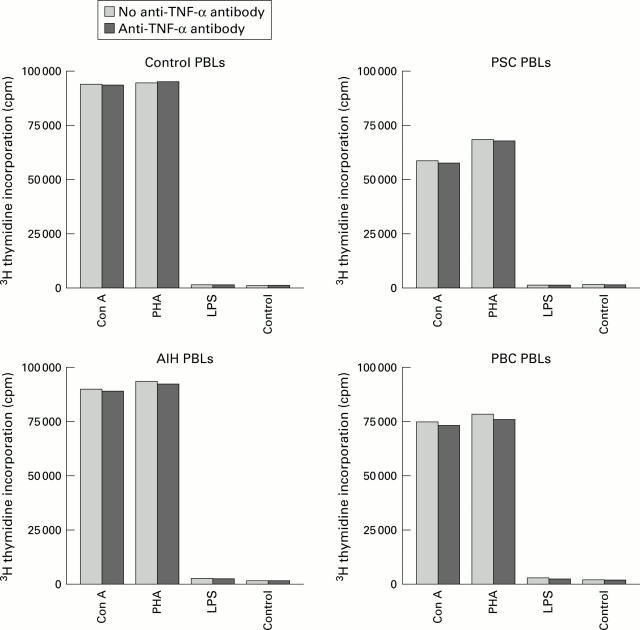
Figure 3 .
Peripheral blood lymphocytes (PBLs) isolated from controls (n=8), primary sclerosing cholangitis (PSC) (n=7), primary biliary cirrhosis (PBC) (n=4), and autoimmune hepatitis (AIH) (n=3) patients showed normal proliferative responses on stimulation with mitogens such as concanavalin A (Con A) and phytohaemagglutinin (PHA) for 72 hours. No difference in responses was observed after pretreatment with anti-tumour necrosis factor (TNF) monoclonal antibodies. Data represent mean counts per minute (cpm) of triplicate cultures. LPS, lipopolysaccharide.
Figure 4 .
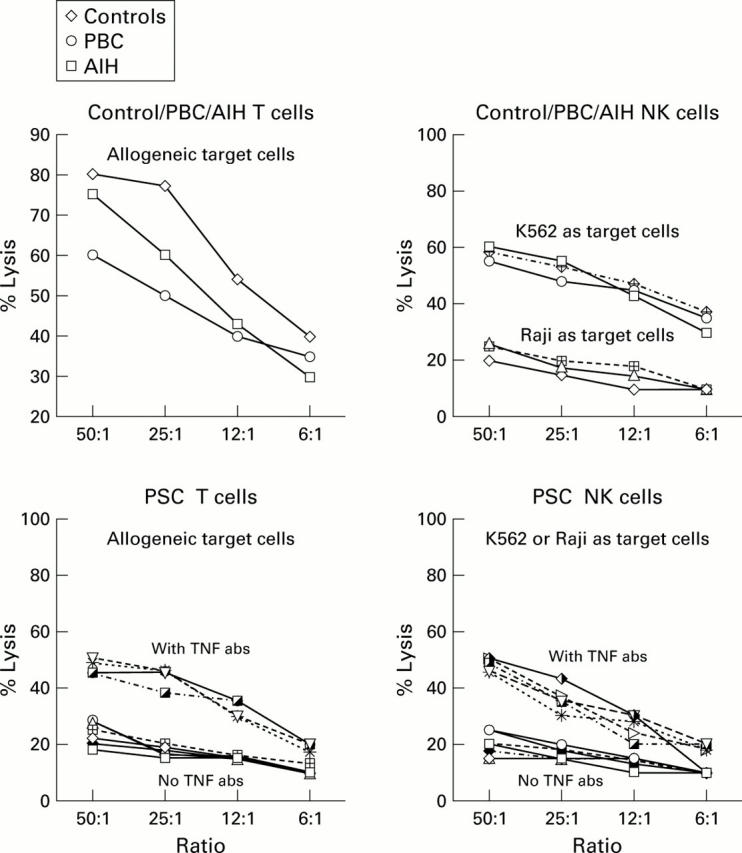
Cytotoxic liver derived lymphocytes (LDLs) (T cells) generated by stimulation against normal allogeneic LDLs from control, primary biliary cirrhosis (PBC), and autoimmune hepatitis (AIH) livers showed high cytotoxic function against allogeneic target cells. In the case of controls, only the average per cent lysis (from eight experiments in normals, four in PBC, and three in AIH) is shown. Cytotoxic LDLs (T cells ) isolated from primary sclerosing cholangitis (PSC) livers (n=7) on the other hand did not show any cytotoxic activity against allogeneic target cells. The cytotoxic activity of T cells was thus completely abolished in the livers of PSC patients (p<0.001). Natural killer (NK) cells (CD56+16+) isolated from control, PBC, and AIH livers showed normal cytotoxic activity against K562 (NK cell sensitive cell line) but not against Raji's (NK resistant cell line) (only mean per cent lysis is shown). NK cells isolated from PSC livers (n=7) on the other hand did not show any cytotoxic activity against K562. NK cell cytotoxic activity was thus completely abolished in the livers of PSC patients (p<0.001). However, overnight treatment of cytotoxic LDLs (T cells) isolated from PSC livers with tumour necrosis factor (TNF) antibodies (abs) (10 mg/ml) showed a moderate increase in cytotoxic activity against allogeneic target cells. Similarly, NK cells (CD56+/16+) showed enhanced cytotoxic activity against K562 (NK cell sensitive cell line).
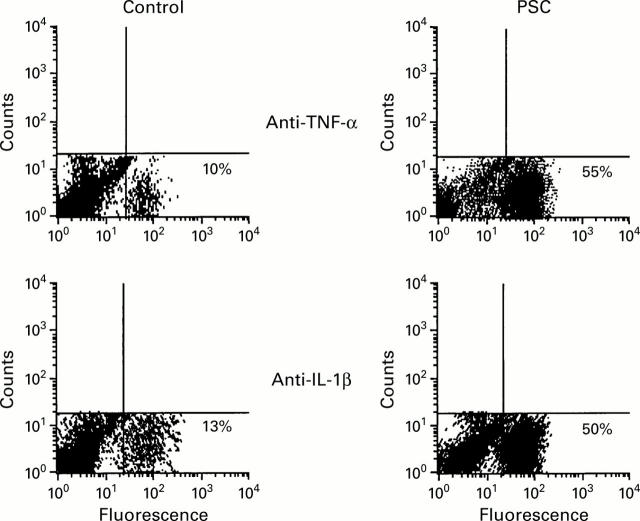
Figure 5 .
Increased immunofluorescent staining of intracellular cytokines tumour necrosis factor α (TNF-α) and interleukin 1β (IL-1β) was seen in freshly isolated liver derived lymphocytes from all seven primary sclerosing cholangitis (PSC) patients but not from controls. A representative picture of this finding from one PSC patient and one normal control is shown. Cells were initially fixed, permeabilised, and stained with fluorescein isothiocynate conjugated anti-TNF/anti-IL-1β antibodies. Unlabelled anti-cytokine monoclonal antibody was used to decrease high background staining.
Figure 6 .
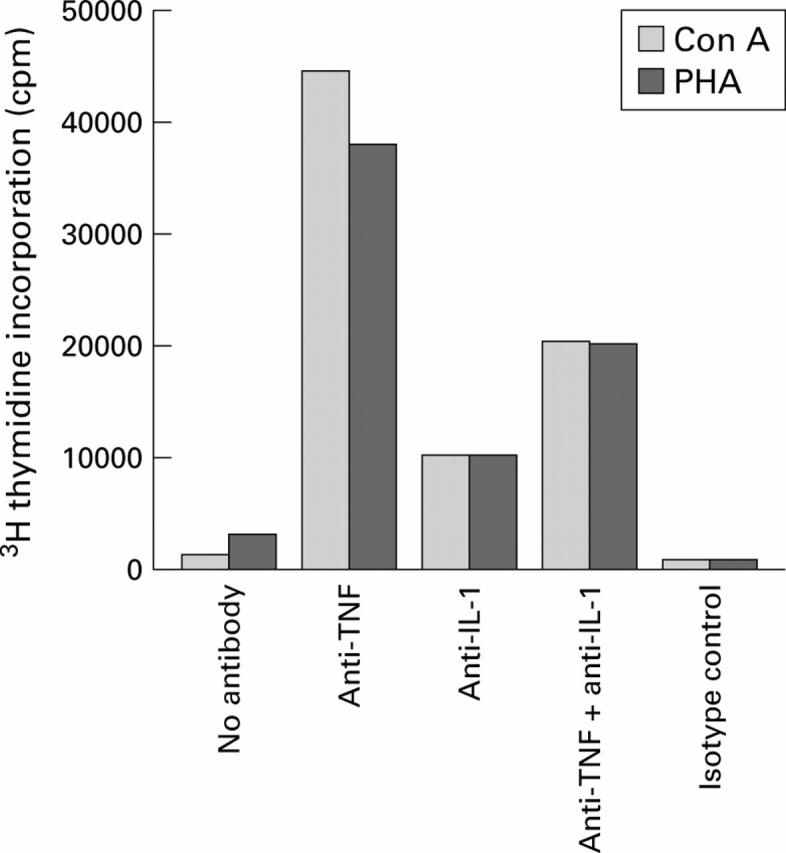
Effect of anti-cytokine antibodies on restoration of diminished proliferative responses to mitogen stimulation by concanavalin A (Con A) and phytohaemagglutinin (PHA). Liver derived lymphocytes (LDLs) from primary sclerosing cholangitis (PSC) livers remained untreated or pretreated with anti-tumour necrosis factor (TNF) monoclonal antibodies (10 µg/ml) and/or interleukin 1 (IL-1) monoclonal antibodies (10 µg/ml), or control monoclonal antibodies (10 µg/ml) overnight. Con A or PHA was added and 3H thymidine incorporation was detected after 72 hours. Anti-TNF monoclonal antibodies alone restored the diminished proliferative responses in the LDLs from PSC patients.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarez F., Berg P. A., Bianchi F. B., Bianchi L., Burroughs A. K., Cancado E. L., Chapman R. W., Cooksley W. G., Czaja A. J., Desmet V. J. International Autoimmune Hepatitis Group Report: review of criteria for diagnosis of autoimmune hepatitis. J Hepatol. 1999 Nov;31(5):929–938. doi: 10.1016/s0168-8278(99)80297-9. [DOI] [PubMed] [Google Scholar]
- Baumgart D. C., Olivier W. A., Reya T., Peritt D., Rombeau J. L., Carding S. R. Mechanisms of intestinal epithelial cell injury and colitis in interleukin 2 (IL2)-deficient mice. Cell Immunol. 1998 Jul 10;187(1):52–66. doi: 10.1006/cimm.1998.1307. [DOI] [PubMed] [Google Scholar]
- Berg P. A., Klein R., Röcken M. Cytokines in primary biliary cirrhosis. Semin Liver Dis. 1997 May;17(2):115–123. doi: 10.1055/s-2007-1007189. [DOI] [PubMed] [Google Scholar]
- Bernal W., Moloney M., Underhill J., Donaldson P. T. Association of tumor necrosis factor polymorphism with primary sclerosing cholangitis. J Hepatol. 1999 Feb;30(2):237–241. doi: 10.1016/s0168-8278(99)80068-3. [DOI] [PubMed] [Google Scholar]
- Boberg K. M., Lundin K. E., Schrumpf E. Etiology and pathogenesis in primary sclerosing cholangitis. Scand J Gastroenterol Suppl. 1994;204:47–58. doi: 10.3109/00365529409103625. [DOI] [PubMed] [Google Scholar]
- Broomé U., Grunewald J., Scheynius A., Olerup O., Hultcrantz R. Preferential V beta3 usage by hepatic T lymphocytes in patients with primary sclerosing cholangitis. J Hepatol. 1997 Mar;26(3):527–534. doi: 10.1016/s0168-8278(97)80417-5. [DOI] [PubMed] [Google Scholar]
- Broomé U., Olsson R., Löf L., Bodemar G., Hultcrantz R., Danielsson A., Prytz H., Sandberg-Gertzén H., Wallerstedt S., Lindberg G. Natural history and prognostic factors in 305 Swedish patients with primary sclerosing cholangitis. Gut. 1996 Apr;38(4):610–615. doi: 10.1136/gut.38.4.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman R. W., Arborgh B. A., Rhodes J. M., Summerfield J. A., Dick R., Scheuer P. J., Sherlock S. Primary sclerosing cholangitis: a review of its clinical features, cholangiography, and hepatic histology. Gut. 1980 Oct;21(10):870–877. doi: 10.1136/gut.21.10.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciacci C., Mahida Y. R., Dignass A., Koizumi M., Podolsky D. K. Functional interleukin-2 receptors on intestinal epithelial cells. J Clin Invest. 1993 Jul;92(1):527–532. doi: 10.1172/JCI116598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope A. P., Aderka D., Wallach D., Kahan M., Chu N. R., Brennan F. M., Feldmann M. Soluble TNF receptor production by activated T lymphocytes: differential effects of acute and chronic exposure to TNF. Immunology. 1995 Jan;84(1):21–30. [PMC free article] [PubMed] [Google Scholar]
- Cope A. P., Liblau R. S., Yang X. D., Congia M., Laudanna C., Schreiber R. D., Probert L., Kollias G., McDevitt H. O. Chronic tumor necrosis factor alters T cell responses by attenuating T cell receptor signaling. J Exp Med. 1997 May 5;185(9):1573–1584. doi: 10.1084/jem.185.9.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope A. P., Londei M., Chu N. R., Cohen S. B., Elliott M. J., Brennan F. M., Maini R. N., Feldmann M. Chronic exposure to tumor necrosis factor (TNF) in vitro impairs the activation of T cells through the T cell receptor/CD3 complex; reversal in vivo by anti-TNF antibodies in patients with rheumatoid arthritis. J Clin Invest. 1994 Aug;94(2):749–760. doi: 10.1172/JCI117394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope A. P. Regulation of autoimmunity by proinflammatory cytokines. Curr Opin Immunol. 1998 Dec;10(6):669–676. doi: 10.1016/s0952-7915(98)80087-3. [DOI] [PubMed] [Google Scholar]
- Fais S., Capobianchi M. R., Pallone F., Di Marco P., Boirivant M., Dianzani F., Torsoli A. Spontaneous release of interferon gamma by intestinal lamina propria lymphocytes in Crohn's disease. Kinetics of in vitro response to interferon gamma inducers. Gut. 1991 Apr;32(4):403–407. doi: 10.1136/gut.32.4.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman R. M., Krueger J., Yourish D., Granelli-Piperno A., Murphy D. P., May L. T., Kupper T. S., Sehgal P. B., Gottlieb A. B. Interleukin 6 is expressed in high levels in psoriatic skin and stimulates proliferation of cultured human keratinocytes. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6367–6371. doi: 10.1073/pnas.86.16.6367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joplin R., Strain A. J., Neuberger J. M. Immuno-isolation and culture of biliary epithelial cells from normal human liver. In Vitro Cell Dev Biol. 1989 Dec;25(12):1189–1192. doi: 10.1007/BF02621273. [DOI] [PubMed] [Google Scholar]
- LaRusso N. F., Wiesner R. H., Ludwig J., MacCarty R. L. Current concepts. Primary sclerosing cholangitis. N Engl J Med. 1984 Apr 5;310(14):899–903. doi: 10.1056/NEJM198404053101407. [DOI] [PubMed] [Google Scholar]
- Leon M. P., Bassendine M. F., Gibbs P., Burt A. D., Thick M., Kirby J. A. Hepatic allograft rejection: regulation of the immunogenicity of human intrahepatic biliary epithelial cells. Liver Transpl Surg. 1996 Jan;2(1):37–45. doi: 10.1002/lt.500020107. [DOI] [PubMed] [Google Scholar]
- Lindor K. D., Wiesner R. H., LaRusso N. F., Homburger H. A. Enhanced autoreactivity of T-lymphocytes in primary sclerosing cholangitis. Hepatology. 1987 Sep-Oct;7(5):884–888. doi: 10.1002/hep.1840070515. [DOI] [PubMed] [Google Scholar]
- Ludwig J., Barham S. S., LaRusso N. F., Elveback L. R., Wiesner R. H., McCall J. T. Morphologic features of chronic hepatitis associated with primary sclerosing cholangitis and chronic ulcerative colitis. Hepatology. 1981 Nov-Dec;1(6):632–640. doi: 10.1002/hep.1840010612. [DOI] [PubMed] [Google Scholar]
- MacDonald T. T., Hutchings P., Choy M. Y., Murch S., Cooke A. Tumour necrosis factor-alpha and interferon-gamma production measured at the single cell level in normal and inflamed human intestine. Clin Exp Immunol. 1990 Aug;81(2):301–305. doi: 10.1111/j.1365-2249.1990.tb03334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuberger J. Primary biliary cirrhosis. Lancet. 1997 Sep 20;350(9081):875–879. doi: 10.1016/S0140-6736(97)05419-6. [DOI] [PubMed] [Google Scholar]
- Niessner M., Volk B. A. Altered Th1/Th2 cytokine profiles in the intestinal mucosa of patients with inflammatory bowel disease as assessed by quantitative reversed transcribed polymerase chain reaction (RT-PCR). Clin Exp Immunol. 1995 Sep;101(3):428–435. doi: 10.1111/j.1365-2249.1995.tb03130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panja A., Goldberg S., Eckmann L., Krishen P., Mayer L. The regulation and functional consequence of proinflammatory cytokine binding on human intestinal epithelial cells. J Immunol. 1998 Oct 1;161(7):3675–3684. [PubMed] [Google Scholar]
- Powrie F. T cells in inflammatory bowel disease: protective and pathogenic roles. Immunity. 1995 Aug;3(2):171–174. doi: 10.1016/1074-7613(95)90086-1. [DOI] [PubMed] [Google Scholar]
- Sadlack B., Merz H., Schorle H., Schimpl A., Feller A. C., Horak I. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell. 1993 Oct 22;75(2):253–261. doi: 10.1016/0092-8674(93)80067-o. [DOI] [PubMed] [Google Scholar]
- Shinmei M., Masuda K., Kikuchi T., Shimomura Y. Interleukin 1, tumor necrosis factor, and interleukin 6 as mediators of cartilage destruction. Semin Arthritis Rheum. 1989 Feb;18(3 Suppl 1):27–32. doi: 10.1016/0049-0172(89)90081-4. [DOI] [PubMed] [Google Scholar]
- Spengler U., Möller A., Jung M. C., Messer G., Zachoval R., Hoffmann R. M., Eisenburg J., Paumgartner G., Riethmüller G., Weiss E. H. T lymphocytes from patients with primary biliary cirrhosis produce reduced amounts of lymphotoxin, tumor necrosis factor and interferon-gamma upon mitogen stimulation. J Hepatol. 1992 May;15(1-2):129–135. doi: 10.1016/0168-8278(92)90024-j. [DOI] [PubMed] [Google Scholar]
- Takemura T., Yoshioka K., Murakami K., Akano N., Okada M., Aya N., Maki S. Cellular localization of inflammatory cytokines in human glomerulonephritis. Virchows Arch. 1994;424(5):459–464. doi: 10.1007/BF00191429. [DOI] [PubMed] [Google Scholar]
- Whiteside T. L., Lasky S., Si L., Van Thiel D. H. Immunologic analysis of mononuclear cells in liver tissues and blood of patients with primary sclerosing cholangitis. Hepatology. 1985 May-Jun;5(3):468–474. doi: 10.1002/hep.1840050321. [DOI] [PubMed] [Google Scholar]
- Wilson A. G., Symons J. A., McDowell T. L., McDevitt H. O., Duff G. W. Effects of a polymorphism in the human tumor necrosis factor alpha promoter on transcriptional activation. Proc Natl Acad Sci U S A. 1997 Apr 1;94(7):3195–3199. doi: 10.1073/pnas.94.7.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]