Abstract
BACKGROUND—Coeliac disease is characterised by increased epithelial renewal associated with a mucosal T cell response to gliadin. Keratinocyte growth factor (KGF) is produced by cytokine activated gut stromal cells and may be a link between mucosal T cell activation in untreated coeliac disease and epithelial hyperplasia. AIMS—To characterise expression of KGF in coeliac disease. METHODS—KGF transcripts in coeliac disease were measured by quantitative competitive reverse transcription-polymerase chain reaction (RT-PCR) and localised using in situ hybridisation. KGF production by gluten reactive CD4+ T cell clones was examined. In addition, KGF transcripts were measured following ex vivo challenge of coeliac biopsies with a peptic-tryptic digest of gliadin. RESULTS—KGF transcripts were elevated in coeliac biopsies compared with normal controls but were not different from non-coeliac disease controls. By in situ hybridisation, KGF mRNA containing cells were present in the upper half of the lamina propria, most abundantly just under the epithelium. There was no signal from cells within the epithelium. Gluten reactive T cell clones did not make KGF. In vitro challenge of coeliac biopsies generated a strong interferon γ response but a specific KGF response could not be detected because of an extremely high number of KGF transcripts in all cultured biopsies. CONCLUSIONS—KGF is overexpressed in coeliac biopsies and in tissues with non-coeliac enteropathy. No evidence was found for KGF production by intraepithelial lymphocytes or lamina propria T cells. Keywords: coeliac disease; keratinocyte growth factor; mRNA expression
Full Text
The Full Text of this article is available as a PDF (219.0 KB).
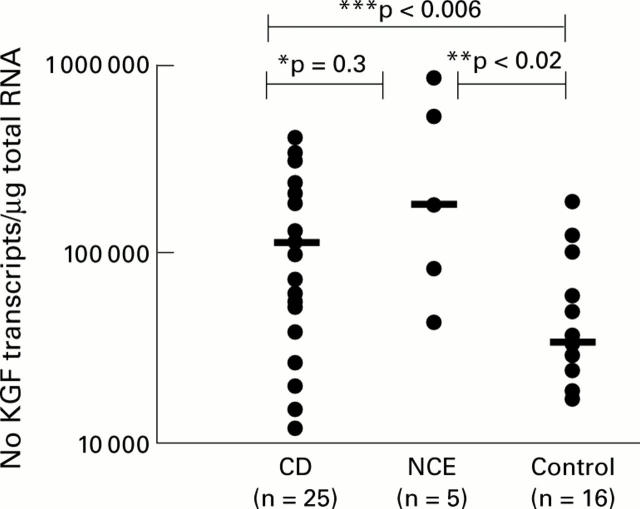
Figure 1 .
Keratinocyte growth factor (KGF) mRNA transcripts in whole small intestinal biopsies from patients with coeliac disease (CD), non-coeliac enteropathy (NCE), and normal controls. Bars represent median KGF mRNA transcripts. ***CD versus controls, p<0.006; **NCE versus controls, p<0.02; *CD versus NCE, p=0.3
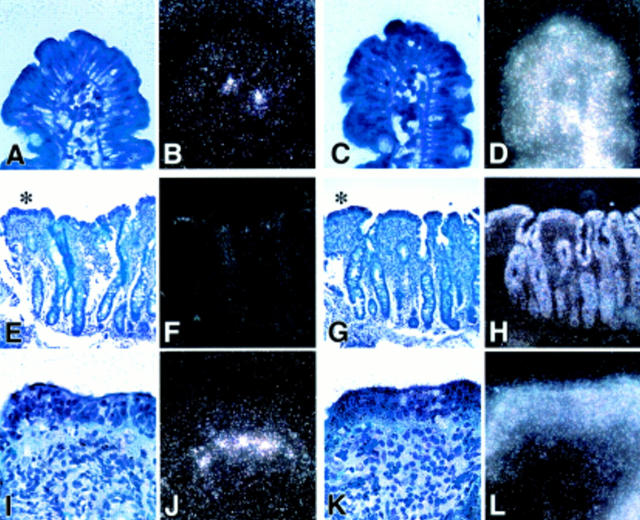
Figure 2 .
Detection of keratinocyte growth factor (KGF) mRNA by in situ hybridisation. (A, B) and (C, D) represent light/dark images of a control section hybridised with antisense probes to KGF mRNA (A, B) and to β-actin (C, D) as a positive control at a magnification of 50×. Very few KGF transcripts were detectable in control sections below the tip of the villi. (E) and (F) show light and dark field images, respectively, at 20× magnification stained for KGF. In (I) and (J) at 50× magnification the flat villi indicated by an asterisk in (E) are shown in more detail. KGF positive hybridising cells are distributed in the subepithelial region of the lamina propria below the tip of the flattened villi. (G, H) (20×) and (K, L) (50×) represent light (G, K) and dark field (H, L) images of the corresponding β-actin staining as a positive control.
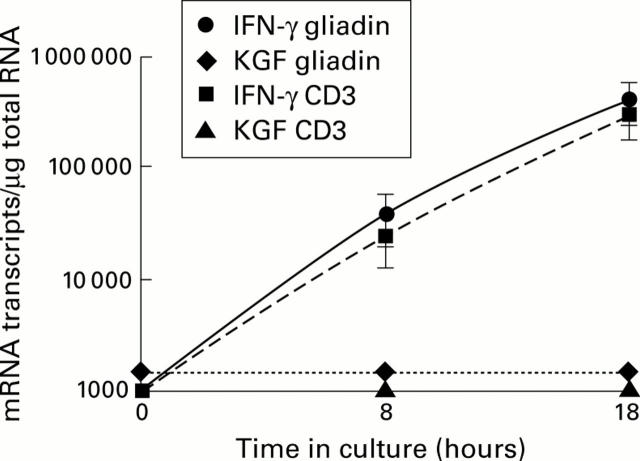
Figure 3 .
Number of keratinocyte growth factor (KGF) and interferon γ (IFN-γ) mRNA transcripts in four gut derived gluten reactive CD4+ T cell clones and lines challenged in vitro with a peptic-tryptic digest of gliadin or anti-CD3 antibodies for eight and 18 hours. Each point at the indicated time represents mean (SEM) values. KGF mRNA expression was below the detection limit (1000 transcripts/µg total RNA) while IFN-γ mRNA transcripts were increased after eight and 18 hours in the presence of gliadin or anti-CD3 antibodies (positive control).
Figure 4 .
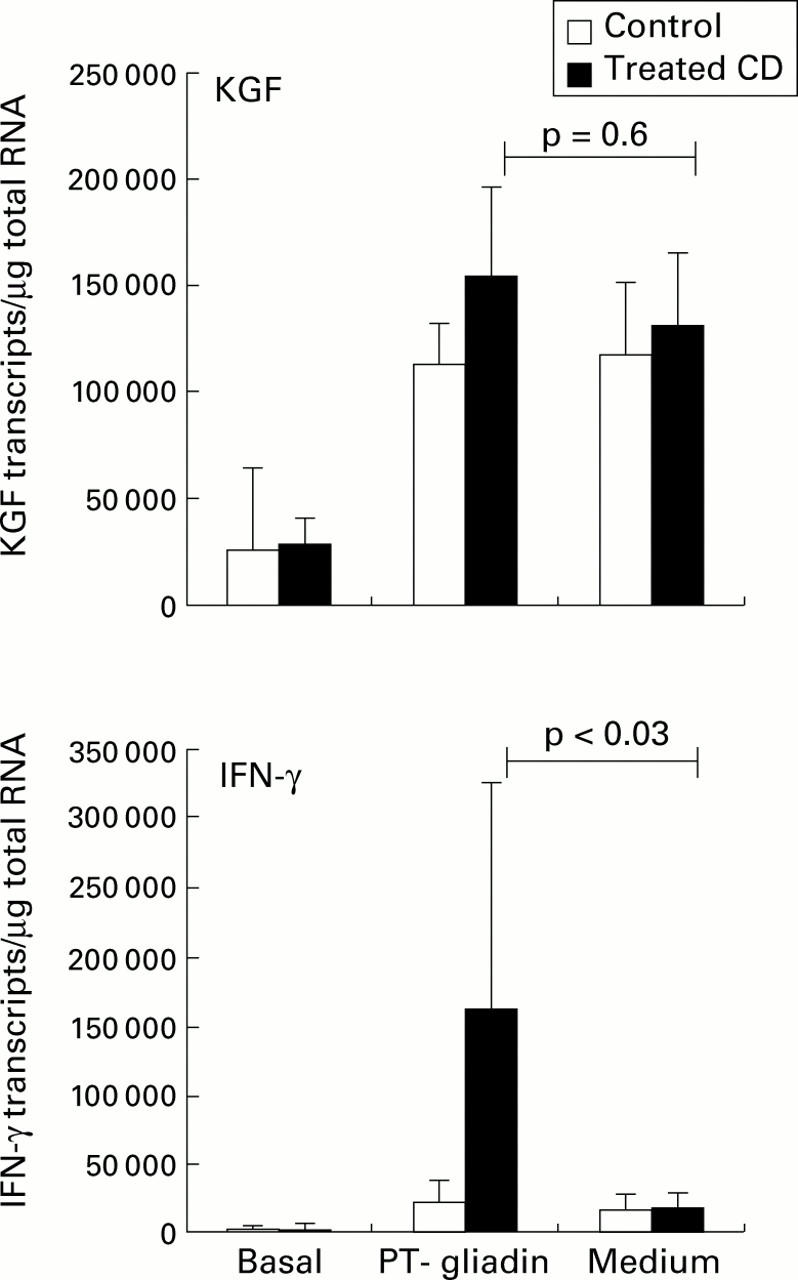
Keratinocyte growth factor (KGF) and interferon γ (IFN-γ) mRNA transcripts measured by competitive quantitative RT-PCR in treated coeliac disease (CD) (n=6) and control biopsies (n=8) challenged in vitro with gliadin. Small intestinal biopsies were cultured for eight hours in the presence or absence of a peptic-tryptic (PT) digest of gliadin (1 mg/ml). Bars represent median (SEM). IFN-γ mRNA transcripts were markedly induced by gliadin in coeliac mucosa compared with biopsies cultured in medium alone (p<0.03). KGF mRNA transcripts were increased in both coeliacs and controls, in the presence and absence of gliadin.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bajaj-Elliott M., Breese E., Poulsom R., Fairclough P. D., MacDonald T. T. Keratinocyte growth factor in inflammatory bowel disease. Increased mRNA transcripts in ulcerative colitis compared with Crohn's disease in biopsies and isolated mucosal myofibroblasts. Am J Pathol. 1997 Nov;151(5):1469–1476. [PMC free article] [PubMed] [Google Scholar]
- Bajaj-Elliott M., Poulsom R., Pender S. L., Wathen N. C., MacDonald T. T. Interactions between stromal cell--derived keratinocyte growth factor and epithelial transforming growth factor in immune-mediated crypt cell hyperplasia. J Clin Invest. 1998 Oct 15;102(8):1473–1480. doi: 10.1172/JCI2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boismenu R., Havran W. L. Modulation of epithelial cell growth by intraepithelial gamma delta T cells. Science. 1994 Nov 18;266(5188):1253–1255. doi: 10.1126/science.7973709. [DOI] [PubMed] [Google Scholar]
- Brauchle M., Madlener M., Wagner A. D., Angermeyer K., Lauer U., Hofschneider P. H., Gregor M., Werner S. Keratinocyte growth factor is highly overexpressed in inflammatory bowel disease. Am J Pathol. 1996 Aug;149(2):521–529. [PMC free article] [PubMed] [Google Scholar]
- Breese E. J., Michie C. A., Nicholls S. W., Murch S. H., Williams C. B., Domizio P., Walker-Smith J. A., MacDonald T. T. Tumor necrosis factor alpha-producing cells in the intestinal mucosa of children with inflammatory bowel disease. Gastroenterology. 1994 Jun;106(6):1455–1466. doi: 10.1016/0016-5085(94)90398-0. [DOI] [PubMed] [Google Scholar]
- Daum S., Bauer U., Foss H. D., Schuppan D., Stein H., Riecken E. O., Ullrich R. Increased expression of mRNA for matrix metalloproteinases-1 and -3 and tissue inhibitor of metalloproteinases-1 in intestinal biopsy specimens from patients with coeliac disease. Gut. 1999 Jan;44(1):17–25. doi: 10.1136/gut.44.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fais S., Maiuri L., Pallone F., De Vincenzi M., De Ritis G., Troncone R., Auricchio S. Gliadin induced changes in the expression of MHC-class II antigens by human small intestinal epithelium. Organ culture studies with coeliac disease mucosa. Gut. 1992 Apr;33(4):472–475. doi: 10.1136/gut.33.4.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch P. W., Cheng A. L. Analysis of the cellular basis of keratinocyte growth factor overexpression in inflammatory bowel disease. Gut. 1999 Dec;45(6):848–855. doi: 10.1136/gut.45.6.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch P. W., Rubin J. S., Miki T., Ron D., Aaronson S. A. Human KGF is FGF-related with properties of a paracrine effector of epithelial cell growth. Science. 1989 Aug 18;245(4919):752–755. doi: 10.1126/science.2475908. [DOI] [PubMed] [Google Scholar]
- Halstensen T. S., Scott H., Brandtzaeg P. Intraepithelial T cells of the TcR gamma/delta+ CD8- and V delta 1/J delta 1+ phenotypes are increased in coeliac disease. Scand J Immunol. 1989 Dec;30(6):665–672. doi: 10.1111/j.1365-3083.1989.tb02474.x. [DOI] [PubMed] [Google Scholar]
- Housley R. M., Morris C. F., Boyle W., Ring B., Biltz R., Tarpley J. E., Aukerman S. L., Devine P. L., Whitehead R. H., Pierce G. F. Keratinocyte growth factor induces proliferation of hepatocytes and epithelial cells throughout the rat gastrointestinal tract. J Clin Invest. 1994 Nov;94(5):1764–1777. doi: 10.1172/JCI117524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung H. C., Eckmann L., Yang S. K., Panja A., Fierer J., Morzycka-Wroblewska E., Kagnoff M. F. A distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion. J Clin Invest. 1995 Jan;95(1):55–65. doi: 10.1172/JCI117676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundin K. E., Scott H., Hansen T., Paulsen G., Halstensen T. S., Fausa O., Thorsby E., Sollid L. M. Gliadin-specific, HLA-DQ(alpha 1*0501,beta 1*0201) restricted T cells isolated from the small intestinal mucosa of celiac disease patients. J Exp Med. 1993 Jul 1;178(1):187–196. doi: 10.1084/jem.178.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald T. T., Bajaj-Elliott M., Pender S. L. T cells orchestrate intestinal mucosal shape and integrity. Immunol Today. 1999 Nov;20(11):505–510. doi: 10.1016/s0167-5699(99)01536-4. [DOI] [PubMed] [Google Scholar]
- MacDonald T. T., Spencer J. Evidence that activated mucosal T cells play a role in the pathogenesis of enteropathy in human small intestine. J Exp Med. 1988 Apr 1;167(4):1341–1349. doi: 10.1084/jem.167.4.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald T. T. The role of activated T lymphocytes in gastrointestinal disease. Clin Exp Allergy. 1990 May;20(3):247–252. doi: 10.1111/j.1365-2222.1990.tb02679.x. [DOI] [PubMed] [Google Scholar]
- Maiuri L., Picarelli A., Boirivant M., Coletta S., Mazzilli M. C., De Vincenzi M., Londei M., Auricchio S. Definition of the initial immunologic modifications upon in vitro gliadin challenge in the small intestine of celiac patients. Gastroenterology. 1996 May;110(5):1368–1378. doi: 10.1053/gast.1996.v110.pm8613040. [DOI] [PubMed] [Google Scholar]
- Monk T., Spencer J., Cerf-Bensussan N., MacDonald T. T. Stimulation of mucosal T cells in situ with anti-CD3 antibody: location of the activated T cells and their distribution within the mucosal micro-environment. Clin Exp Immunol. 1988 Nov;74(2):216–222. [PMC free article] [PubMed] [Google Scholar]
- Murphy M. S. Growth factors and the gastrointestinal tract. Nutrition. 1998 Oct;14(10):771–774. doi: 10.1016/s0899-9007(98)00081-1. [DOI] [PubMed] [Google Scholar]
- Nilsen E. M., Jahnsen F. L., Lundin K. E., Johansen F. E., Fausa O., Sollid L. M., Jahnsen J., Scott H., Brandtzaeg P. Gluten induces an intestinal cytokine response strongly dominated by interferon gamma in patients with celiac disease. Gastroenterology. 1998 Sep;115(3):551–563. doi: 10.1016/s0016-5085(98)70134-9. [DOI] [PubMed] [Google Scholar]
- Revised criteria for diagnosis of coeliac disease. Report of Working Group of European Society of Paediatric Gastroenterology and Nutrition. Arch Dis Child. 1990 Aug;65(8):909–911. doi: 10.1136/adc.65.8.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer J., Isaacson P. G., MacDonald T. T., Thomas A. J., Walker-Smith J. A. Gamma/delta T cells and the diagnosis of coeliac disease. Clin Exp Immunol. 1991 Jul;85(1):109–113. doi: 10.1111/j.1365-2249.1991.tb05690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasunaga Y., Shinomura Y., Kanayama S., Higashimoto Y., Yabu M., Miyazaki Y., Kondo S., Murayama Y., Nishibayashi H., Kitamura S. Increased production of interleukin 1 beta and hepatocyte growth factor may contribute to foveolar hyperplasia in enlarged fold gastritis. Gut. 1996 Dec;39(6):787–794. doi: 10.1136/gut.39.6.787. [DOI] [PMC free article] [PubMed] [Google Scholar]