Abstract
BACKGROUND—The primary pathology of Hirschsprung's disease (HD) is a congenital absence of ganglion cells in the caudal most gut. The spastic aganglionic bowel is often innervated by a network of hypertrophied nerve fibres. Recently, mutations of SOX10 have been identified in patients with HD but only in those with Waardenburg-Shah syndrome. AIMS—To understand the molecular basis for the pathogenesis of HD we intended to determine the specific cell lineages in the enteric nervous system which normally express SOX10 but are affected in disease conditions. METHODS—We studied colon biopsies from 10 non-syndromic HD patients, aged three months to four years, and 10 age matched patients without HD as normal controls. The absence of mutation in the SOX10 gene of HD patients was confirmed by DNA sequencing. Expression and cellular distribution of SOX10 in bowel segments of normal and HD infants were examined by reverse transcription-polymerase chain reaction and in situ hybridisation. RESULTS—We found that in normal infants and normoganglionic bowel segments of HD patients, SOX10 was expressed in both neurones and glia of the enteric plexuses and in the nerves among the musculature in normal colon. In the aganglionic bowel segments of patients, SOX10 expression was consistently lower and was found to be associated with the hypertrophic nerve trunks in the muscle and extrinsic nerves in the serosa. CONCLUSION—We conclude that SOX10 is normally required postnatally in the functional maintenance of the entire enteric nervous system, including neurones and glia. In non-syndromic HD patients who do not have the SOX10 mutation, the SOX10 gene expressed in the sacral region may be involved in the pathogenesis of the abnormal nerve trunks through interaction with other factors. Keywords: SOX10; polymorphism; enteric nervous system; Hirschsprung's disease; colon; neurocristopathy
Full Text
The Full Text of this article is available as a PDF (900.4 KB).
Figure 1 .
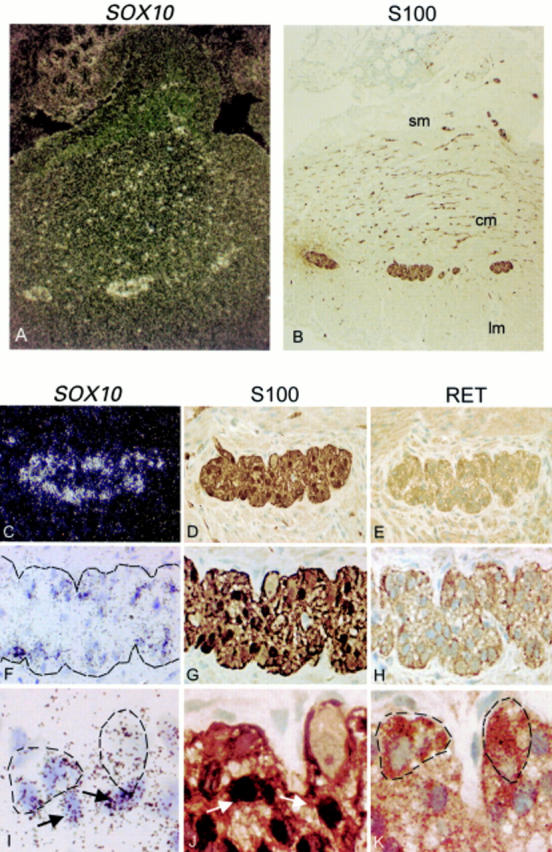
Expression of SOX10 in both neurones and glia of enteric plexuses in the colon of normal infants. (A) Dark field illumination of a cross section of normal colon showing strong in situ hybridisation signals in myenteric plexuses and in the nerves in the longitudinal and circular muscular layers. (B) Bright field illumination of an adjacent section showing S100 immunoreactivity in the SOX10 positive cells. (C) Dark field illumination and (F, I) bright field illumination of a myenteric plexus hybridised in situ with a SOX10 probe. (D, G, J) Immunohistochemistry of an adjacent section with S100 antibody showing that the glial cells present in the plexus are stained. (E, H, K) Immunohistochemistry of another adjacent section with RET antibody showing that the neurones and nerves in the plexus are stained. For comparison, in I, J, and K, the same glial cells are marked with arrows and the neurones are circled by broken lines. Both glial cells immunoreactive for S100 and neurones immunoreactive for RET had positive SOX10 hybridisation signals. Original magnification: (A, B) ×100; (C, D, E) ×400; (F, G, H) ×500; (I, J, K) ×1000. Sm, submucosa; cm, circular muscle; lm, longitudinal muscle.
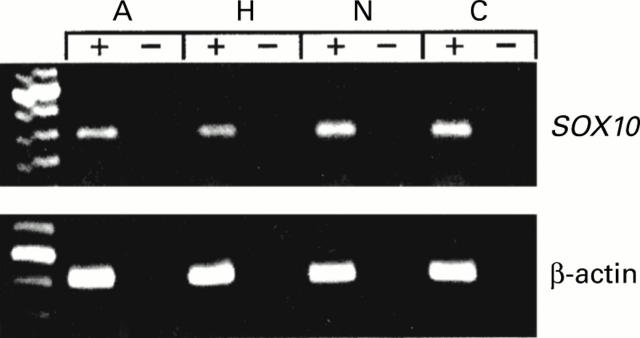
Figure 2 .
Expression of SOX10 in the aganglionic (A), hypoganglionic (H), and normoganglionic (N) colon segments of a Hirschsprung's disease (HD) patient examined by reverse transcription-polymerase chain reaction (RT-PCR) in the presence (+) and absence (−) of reverse transcriptase. Expression of SOX10 in normal control colon tissue (C) is also shown. The expected SOX10 fragment of about 250 bp was amplified from normal control and HD patient samples. As a control the β-actin band of about 300 bp was also amplified from all samples. No DNA fragment could be amplified from cDNA samples that lacked reverse transcriptase (−) indicating that the amplified bands were derived from mRNA.
Figure 3 .
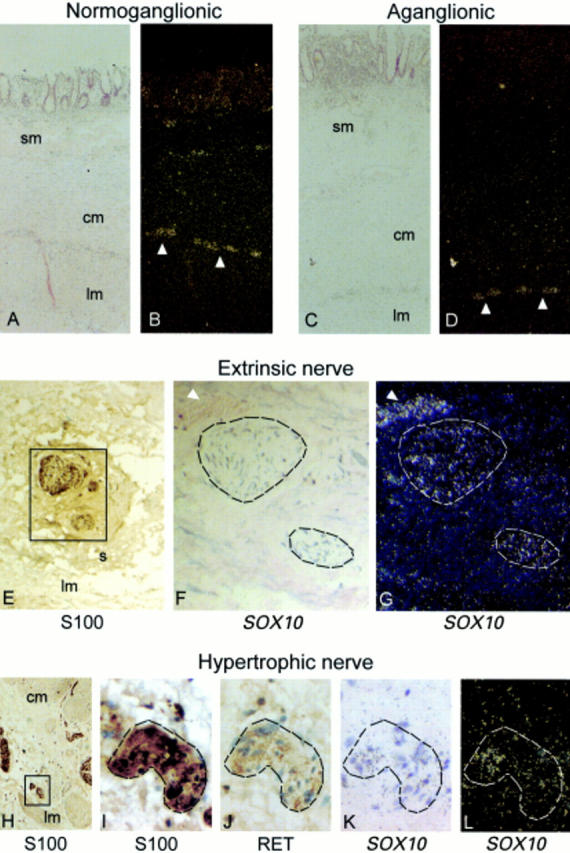
Expression of SOX10 in the normoganglionic (A, B) and aganglionic (C-L) colon segments of a Hirschsprung's disease patient examined by in situ hybridisation. SOX10 is expressed in myenteric plexuses (arrowhead) and nerves in the circular and longitudinal muscles in the normoganglionic colon (A, bright field; B, dark field). In the aganglionic colon, SOX10 is expressed in the hypertrophic nerve trunks and in nerves in the musculature (C, K, bright field; D, L, dark field). Immunohistochemistry on adjacent sections using S100 (H, I) and RET (J) antibodies confirms that the hypertrophic nerves contain nerve fibres and glial cells but no neurones. The SOX10 positive signals are found in the glia and nerve bundles. In addition, SOX10 is also weakly expressed in extrinsic nerves (F, bright field; G, dark field; arrowhead, blood vessel) in the serosa. Immunohistochemistry on an adjacent section shows that the extrinsic nerve is immunoreactive for S100 antibody (E). Original magnification: (A-D) ×100; (E) ×200; (F, G, I-L) ×500; (H) ×100. Sm, submucosa; cm, circular muscle; lm, longitudinal muscle; s, serosa.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amiel J., Attié T., Jan D., Pelet A., Edery P., Bidaud C., Lacombe D., Tam P., Simeoni J., Flori E. Heterozygous endothelin receptor B (EDNRB) mutations in isolated Hirschsprung disease. Hum Mol Genet. 1996 Mar;5(3):355–357. doi: 10.1093/hmg/5.3.355. [DOI] [PubMed] [Google Scholar]
- Attié T., Till M., Pelet A., Amiel J., Edery P., Boutrand L., Munnich A., Lyonnet S. Mutation of the endothelin-receptor B gene in Waardenburg-Hirschsprung disease. Hum Mol Genet. 1995 Dec;4(12):2407–2409. doi: 10.1093/hmg/4.12.2407. [DOI] [PubMed] [Google Scholar]
- Auricchio A., Casari G., Staiano A., Ballabio A. Endothelin-B receptor mutations in patients with isolated Hirschsprung disease from a non-inbred population. Hum Mol Genet. 1996 Mar;5(3):351–354. doi: 10.1093/hmg/5.3.351. [DOI] [PubMed] [Google Scholar]
- Bolk S., Pelet A., Hofstra R. M., Angrist M., Salomon R., Croaker D., Buys C. H., Lyonnet S., Chakravarti A. A human model for multigenic inheritance: phenotypic expression in Hirschsprung disease requires both the RET gene and a new 9q31 locus. Proc Natl Acad Sci U S A. 2000 Jan 4;97(1):268–273. doi: 10.1073/pnas.97.1.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondurand N., Kobetz A., Pingault V., Lemort N., Encha-Razavi F., Couly G., Goerich D. E., Wegner M., Abitbol M., Goossens M. Expression of the SOX10 gene during human development. FEBS Lett. 1998 Aug 7;432(3):168–172. doi: 10.1016/s0014-5793(98)00843-6. [DOI] [PubMed] [Google Scholar]
- Bondurand N., Pingault V., Goerich D. E., Lemort N., Sock E., Le Caignec C., Wegner M., Goossens M. Interaction among SOX10, PAX3 and MITF, three genes altered in Waardenburg syndrome. Hum Mol Genet. 2000 Aug 12;9(13):1907–1917. doi: 10.1093/hmg/9.13.1907. [DOI] [PubMed] [Google Scholar]
- Brand M., Le Moullec J. M., Corvol P., Gasc J. M. Ontogeny of endothelins-1 and -3, their receptors, and endothelin converting enzyme-1 in the early human embryo. J Clin Invest. 1998 Feb 1;101(3):549–559. doi: 10.1172/JCI524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns A. J., Champeval D., Le Douarin N. M. Sacral neural crest cells colonise aganglionic hindgut in vivo but fail to compensate for lack of enteric ganglia. Dev Biol. 2000 Mar 1;219(1):30–43. doi: 10.1006/dbio.1999.9592. [DOI] [PubMed] [Google Scholar]
- Burns A. J., Douarin N. M. The sacral neural crest contributes neurons and glia to the post-umbilical gut: spatiotemporal analysis of the development of the enteric nervous system. Development. 1998 Nov;125(21):4335–4347. doi: 10.1242/dev.125.21.4335. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Doray B., Salomon R., Amiel J., Pelet A., Touraine R., Billaud M., Attié T., Bachy B., Munnich A., Lyonnet S. Mutation of the RET ligand, neurturin, supports multigenic inheritance in Hirschsprung disease. Hum Mol Genet. 1998 Sep;7(9):1449–1452. doi: 10.1093/hmg/7.9.1449. [DOI] [PubMed] [Google Scholar]
- Edery P., Attié T., Amiel J., Pelet A., Eng C., Hofstra R. M., Martelli H., Bidaud C., Munnich A., Lyonnet S. Mutation of the endothelin-3 gene in the Waardenburg-Hirschsprung disease (Shah-Waardenburg syndrome). Nat Genet. 1996 Apr;12(4):442–444. doi: 10.1038/ng0496-442. [DOI] [PubMed] [Google Scholar]
- Erickson C. A., Goins T. L. Sacral neural crest cell migration to the gut is dependent upon the migratory environment and not cell-autonomous migratory properties. Dev Biol. 2000 Mar 1;219(1):79–97. doi: 10.1006/dbio.1999.9597. [DOI] [PubMed] [Google Scholar]
- Fewtrell M. S., Tam P. K., Thomson A. H., Fitchett M., Currie J., Huson S. M., Mulligan L. M. Hirschsprung's disease associated with a deletion of chromosome 10 (q11.2q21.2): a further link with the neurocristopathies? J Med Genet. 1994 Apr;31(4):325–327. doi: 10.1136/jmg.31.4.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frigo G. M., Del Tacca M., Lecchini S., Crema A. Some observations on the intrinsic nervous mechanism in Hirschsprung's disease. Gut. 1973 Jan;14(1):35–40. doi: 10.1136/gut.14.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon M. D. Genes and lineages in the formation of the enteric nervous system. Curr Opin Neurobiol. 1997 Feb;7(1):101–109. doi: 10.1016/s0959-4388(97)80127-4. [DOI] [PubMed] [Google Scholar]
- Giaroni C., De Ponti F., Cosentino M., Lecchini S., Frigo G. Plasticity in the enteric nervous system. Gastroenterology. 1999 Dec;117(6):1438–1458. doi: 10.1016/s0016-5085(99)70295-7. [DOI] [PubMed] [Google Scholar]
- Goyal R. K., Hirano I. The enteric nervous system. N Engl J Med. 1996 Apr 25;334(17):1106–1115. doi: 10.1056/NEJM199604253341707. [DOI] [PubMed] [Google Scholar]
- Herbarth B., Pingault V., Bondurand N., Kuhlbrodt K., Hermans-Borgmeyer I., Puliti A., Lemort N., Goossens M., Wegner M. Mutation of the Sry-related Sox10 gene in Dominant megacolon, a mouse model for human Hirschsprung disease. Proc Natl Acad Sci U S A. 1998 Apr 28;95(9):5161–5165. doi: 10.1073/pnas.95.9.5161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoehner J. C., Wester T., Påhlman S., Olsen L. Alterations in neurotrophin and neurotrophin-receptor localization in Hirschsprung's disease. J Pediatr Surg. 1996 Nov;31(11):1524–1529. doi: 10.1016/s0022-3468(96)90170-0. [DOI] [PubMed] [Google Scholar]
- Hofstra R. M., Osinga J., Tan-Sindhunata G., Wu Y., Kamsteeg E. J., Stulp R. P., van Ravenswaaij-Arts C., Majoor-Krakauer D., Angrist M., Chakravarti A. A homozygous mutation in the endothelin-3 gene associated with a combined Waardenburg type 2 and Hirschsprung phenotype (Shah-Waardenburg syndrome). Nat Genet. 1996 Apr;12(4):445–447. doi: 10.1038/ng0496-445. [DOI] [PubMed] [Google Scholar]
- Inoue K., Tanabe Y., Lupski J. R. Myelin deficiencies in both the central and the peripheral nervous systems associated with a SOX10 mutation. Ann Neurol. 1999 Sep;46(3):313–318. doi: 10.1002/1531-8249(199909)46:3<313::aid-ana6>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Kobayashi H., O'Briain D. S., Puri P. Nerve growth factor receptor immunostaining suggests an extrinsic origin for hypertrophic nerves in Hirschsprung's disease. Gut. 1994 Nov;35(11):1605–1607. doi: 10.1136/gut.35.11.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlbrodt K., Herbarth B., Sock E., Hermans-Borgmeyer I., Wegner M. Sox10, a novel transcriptional modulator in glial cells. J Neurosci. 1998 Jan 1;18(1):237–250. doi: 10.1523/JNEUROSCI.18-01-00237.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda T., Ueda M., Nakano M., Saeki M. Altered production of nerve growth factor in aganglionic intestines. J Pediatr Surg. 1994 Feb;29(2):288–293. doi: 10.1016/0022-3468(94)90334-4. [DOI] [PubMed] [Google Scholar]
- Kusafuka T., Wang Y., Puri P. Novel mutations of the endothelin-B receptor gene in isolated patients with Hirschsprung's disease. Hum Mol Genet. 1996 Mar;5(3):347–349. doi: 10.1093/hmg/5.3.347. [DOI] [PubMed] [Google Scholar]
- Lang D., Chen F., Milewski R., Li J., Lu M. M., Epstein J. A. Pax3 is required for enteric ganglia formation and functions with Sox10 to modulate expression of c-ret. J Clin Invest. 2000 Oct;106(8):963–971. doi: 10.1172/JCI10828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M., Goodall J., Verastegui C., Ballotti R., Goding C. R. Direct regulation of the Microphthalmia promoter by Sox10 links Waardenburg-Shah syndrome (WS4)-associated hypopigmentation and deafness to WS2. J Biol Chem. 2000 Dec 1;275(48):37978–37983. doi: 10.1074/jbc.M003816200. [DOI] [PubMed] [Google Scholar]
- Liu Q., Melnikova I. N., Hu M., Gardner P. D. Cell type-specific activation of neuronal nicotinic acetylcholine receptor subunit genes by Sox10. J Neurosci. 1999 Nov 15;19(22):9747–9755. doi: 10.1523/JNEUROSCI.19-22-09747.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyonnet S., Bolino A., Pelet A., Abel L., Nihoul-Fékété C., Briard M. L., Mok-Siu V., Kaariainen H., Martucciello G., Lerone M. A gene for Hirschsprung disease maps to the proximal long arm of chromosome 10. Nat Genet. 1993 Aug;4(4):346–350. doi: 10.1038/ng0893-346. [DOI] [PubMed] [Google Scholar]
- Pingault V., Bondurand N., Kuhlbrodt K., Goerich D. E., Préhu M. O., Puliti A., Herbarth B., Hermans-Borgmeyer I., Legius E., Matthijs G. SOX10 mutations in patients with Waardenburg-Hirschsprung disease. Nat Genet. 1998 Feb;18(2):171–173. doi: 10.1038/ng0298-171. [DOI] [PubMed] [Google Scholar]
- Pomeranz H. D., Gershon M. D. Colonization of the avian hindgut by cells derived from the sacral neural crest. Dev Biol. 1990 Feb;137(2):378–394. doi: 10.1016/0012-1606(90)90262-h. [DOI] [PubMed] [Google Scholar]
- Pomeranz H. D., Rothman T. P., Gershon M. D. Colonization of the post-umbilical bowel by cells derived from the sacral neural crest: direct tracing of cell migration using an intercalating probe and a replication-deficient retrovirus. Development. 1991 Mar;111(3):647–655. doi: 10.1242/dev.111.3.647. [DOI] [PubMed] [Google Scholar]
- Potterf S. B., Furumura M., Dunn K. J., Arnheiter H., Pavan W. J. Transcription factor hierarchy in Waardenburg syndrome: regulation of MITF expression by SOX10 and PAX3. Hum Genet. 2000 Jul;107(1):1–6. doi: 10.1007/s004390000328. [DOI] [PubMed] [Google Scholar]
- Puffenberger E. G., Hosoda K., Washington S. S., Nakao K., deWit D., Yanagisawa M., Chakravart A. A missense mutation of the endothelin-B receptor gene in multigenic Hirschsprung's disease. Cell. 1994 Dec 30;79(7):1257–1266. doi: 10.1016/0092-8674(94)90016-7. [DOI] [PubMed] [Google Scholar]
- Robertson K., Mason I., Hall S. Hirschsprung's disease: genetic mutations in mice and men. Gut. 1997 Oct;41(4):436–441. doi: 10.1136/gut.41.4.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon R., Attié T., Pelet A., Bidaud C., Eng C., Amiel J., Sarnacki S., Goulet O., Ricour C., Nihoul-Fékété C. Germline mutations of the RET ligand GDNF are not sufficient to cause Hirschsprung disease. Nat Genet. 1996 Nov;14(3):345–347. doi: 10.1038/ng1196-345. [DOI] [PubMed] [Google Scholar]
- Sancandi M., Ceccherini I., Costa M., Fava M., Chen B., Wu Y., Hofstra R., Laurie T., Griffths M., Burge D. Incidence of RET mutations in patients with Hirschsprung's disease. J Pediatr Surg. 2000 Jan;35(1):139–143. doi: 10.1016/s0022-3468(00)80031-7. [DOI] [PubMed] [Google Scholar]
- Serbedzija G. N., Burgan S., Fraser S. E., Bronner-Fraser M. Vital dye labelling demonstrates a sacral neural crest contribution to the enteric nervous system of chick and mouse embryos. Development. 1991 Apr;111(4):857–866. doi: 10.1242/dev.111.4.857. [DOI] [PubMed] [Google Scholar]
- Southard-Smith E. M., Angrist M., Ellison J. S., Agarwala R., Baxevanis A. D., Chakravarti A., Pavan W. J. The Sox10(Dom) mouse: modeling the genetic variation of Waardenburg-Shah (WS4) syndrome. Genome Res. 1999 Mar;9(3):215–225. [PubMed] [Google Scholar]
- Southard-Smith E. M., Kos L., Pavan W. J. Sox10 mutation disrupts neural crest development in Dom Hirschsprung mouse model. Nat Genet. 1998 Jan;18(1):60–64. doi: 10.1038/ng0198-60. [DOI] [PubMed] [Google Scholar]
- Tam P. K., Boyd G. P. Origin, course, and endings of abnormal enteric nerve fibres in Hirschsprung's disease defined by whole-mount immunohistochemistry. J Pediatr Surg. 1990 Apr;25(4):457–461. doi: 10.1016/0022-3468(90)90394-o. [DOI] [PubMed] [Google Scholar]
- Touraine R. L., Attié-Bitach T., Manceau E., Korsch E., Sarda P., Pingault V., Encha-Razavi F., Pelet A., Augé J., Nivelon-Chevallier A. Neurological phenotype in Waardenburg syndrome type 4 correlates with novel SOX10 truncating mutations and expression in developing brain. Am J Hum Genet. 2000 Apr 4;66(5):1496–1503. doi: 10.1086/302895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderwinden J. M., De Laet M. H., Schiffmann S. N., Mailleux P., Lowenstein C. J., Snyder S. H., Vanderhaeghen J. J. Nitric oxide synthase distribution in the enteric nervous system of Hirschsprung's disease. Gastroenterology. 1993 Oct;105(4):969–973. doi: 10.1016/0016-5085(93)90938-9. [DOI] [PubMed] [Google Scholar]
- Verastegui C., Bille K., Ortonne J. P., Ballotti R. Regulation of the microphthalmia-associated transcription factor gene by the Waardenburg syndrome type 4 gene, SOX10. J Biol Chem. 2000 Oct 6;275(40):30757–30760. doi: 10.1074/jbc.C000445200. [DOI] [PubMed] [Google Scholar]
- Wilkinson D. G., Nieto M. A. Detection of messenger RNA by in situ hybridization to tissue sections and whole mounts. Methods Enzymol. 1993;225:361–373. doi: 10.1016/0076-6879(93)25025-w. [DOI] [PubMed] [Google Scholar]
- Wright E. M., Snopek B., Koopman P. Seven new members of the Sox gene family expressed during mouse development. Nucleic Acids Res. 1993 Feb 11;21(3):744–744. doi: 10.1093/nar/21.3.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin L., Barone V., Seri M., Bolino A., Bocciardi R., Ceccherini I., Pasini B., Tocco T., Lerone M., Cywes S. Heterogeneity and low detection rate of RET mutations in Hirschsprung disease. Eur J Hum Genet. 1994;2(4):272–280. doi: 10.1159/000472371. [DOI] [PubMed] [Google Scholar]
- Young H. M., Hearn C. J., Ciampoli D., Southwell B. R., Brunet J. F., Newgreen D. F. A single rostrocaudal colonization of the rodent intestine by enteric neuron precursors is revealed by the expression of Phox2b, Ret, and p75 and by explants grown under the kidney capsule or in organ culture. Dev Biol. 1998 Oct 1;202(1):67–84. doi: 10.1006/dbio.1998.8987. [DOI] [PubMed] [Google Scholar]
- Zhou Q., Wang S., Anderson D. J. Identification of a novel family of oligodendrocyte lineage-specific basic helix-loop-helix transcription factors. Neuron. 2000 Feb;25(2):331–343. doi: 10.1016/s0896-6273(00)80898-3. [DOI] [PubMed] [Google Scholar]