Abstract
BACKGROUND—Erythropoietin (Epo) receptors are present on enterocytes of fetal and neonatal small bowel but the role of Epo in the bowel is not known. AIMS—We tested the following hypotheses: (1) enterally dosed Epo is absorbed from the intestines of neonatal rats, (2) Epo acts as a trophic factor in developing small bowel, and (3) the trophic effects of Epo are dependant on the route of administration. METHODS—The dose dependant effects of enterally dosed recombinant human erythropoietin (rEpo 0-1000 U/kg/day) were studied in artificially raised rat pups and compared with dam raised controls and dam raised pups given rEpo in rat milk. After one week, reticulocyte counts, haematocrits, and plasma Epo concentrations were measured, and calibrated morphometric measurements of villi were performed. The effects of route of rEpo administration (enteral v parenteral) on erythropoiesis, bowel growth, and disaccharidase activity were studied in nursing pups treated for one and two weeks. RESULTS—Serum Epo concentrations ranged from undetectable (<0.6 mU/ml) to 8.4 mU/ml in control and enterally dosed pups (median 1.8 mU/ml), and from 4.9 to 82.3 mU/ml (median 20.4 mU/ml) in parenterally dosed animals. No increase in haematocrit or reticulocyte count was noted in enterally treated pups compared with controls after up to two weeks of treatment. Small bowel length was greater in rEpo treated pups, and a dose dependent increase in villus surface area which was independent of the route of dosing and associated with increased BrdU uptake was found. CONCLUSIONS—rEpo is not enterally absorbed in an intact and functional form from the intestines of neonatal rat pups. Thus enterally dosed rEpo has no erythropoietic effects. However, rEpo acts as a trophic factor in developing rat small bowel whether given enterally or parenterally. Keywords: erythropoietin; enterocytes; breast milk; development; neonate; rat
Full Text
The Full Text of this article is available as a PDF (280.6 KB).
Figure 1 .

Erythropoietin (Epo) concentrations and haematocrit values from artificially raised and nursing rat pups. (A) Epo concentrations in the different treatment groups. The first three groups were artificially raised and received exogenous recombinant human erythropoietin (rEpo) as follows: control (C), 0 U/kg/day; low dose, 200 U/kg/day; and high dose, 1000 U/kg/day. The nursing control pups received no exogenous rEpo while the nursing Epo treated group received rEpo contained in mothers' milk. The top, bottom, and line through the middle of the box plot indicate the 75th, 25th, and 50th percentiles (median), respectively. The whiskers on the bottom extend from the 10th to the 90th percentiles. Mean values for each group are denoted by the filled box. (B) Haematocrit values in the treatment groups defined above.
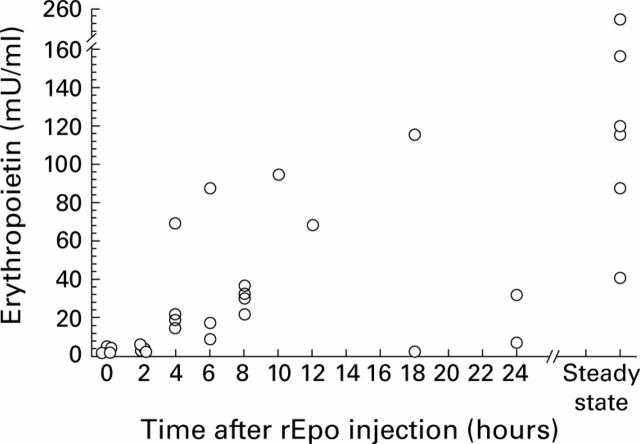
Figure 2 .
Erythropoietin (Epo) concentrations in rat milk following timed recombinant human erythropoietin (rEpo) injection. Epo concentrations (mU/ml) are shown versus time after a single injection of 200 U rEpo intraperitoneally. The right hand side of the X axis shows Epo concentrations drawn 24 hours after the last dose of rEpo to animals that had received daily doses of 200 U rEpo intraperitoneally for seven days.
Figure 3 .

Erythropoietin (Epo) concentrations and haematocrit values from nursing rat pups. (A) Epo concentrations in the treatment groups: control (no exogenous recombinant human erythropoietin (rEpo)), one and two weeks of therapy; PO rEpo (dam received daily dose of 200 U rEpo intraperitoneally), one and two weeks of therapy; and SC rEpo (pups received daily dose of 200 U/kg/day subcutaneously), one and two weeks of therapy. (B) Haematocrit values in the same treatment groups. Box plots as described in fig 1. *p<0.05, **p<0.001.
Figure 4 .

Morphometric measurements of the small bowel from control pups treated with no exogenous recombinant human erythropoietin (rEpo) compared with enteral and parenterally treated pups. Measurements are mean (SEM) for villus surface area (top), villus length (middle), and crypt depth (bottom). Measurements for each treatment group (control (C), no exogenous rEpo; PO, dam received daily dose of 200 U rEpo intraperitoneally; SC, pups received daily dose of 200 U/kg/day rEpo subcutaneously) are compared by small bowel region (duodenum, jejunum, and ileum). *p<0.05, **p<0.01.
Figure 5 .
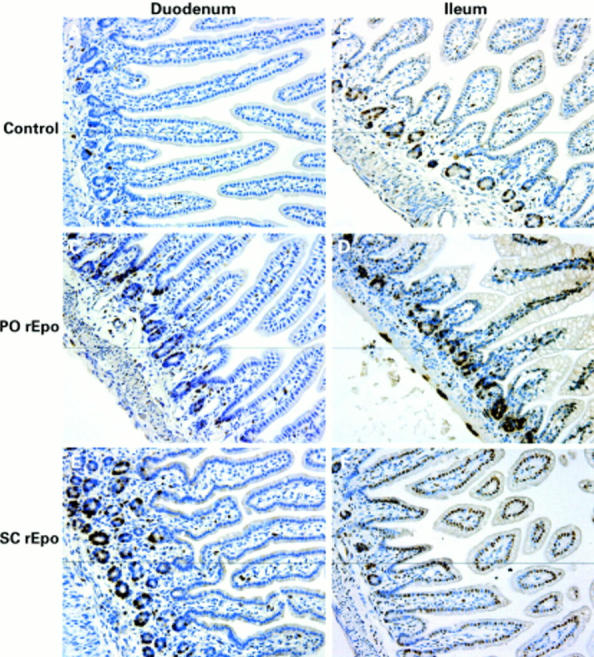
Bromodeoxyuridine (BrdU) incorporation in neonatal rat small bowel. All images were taken at an original magnification of 400×. (A, B) Duodenum and ileum from control animals; (C, D) duodenum and ileum from animals treated with recombinant human erythropoietin intraperitoneally (PO rEpo); and (E, F) duodenum and ileum from subcutaneous rEpo (SC rEpo) treated animals. Brown staining shows BrdU positive cells.
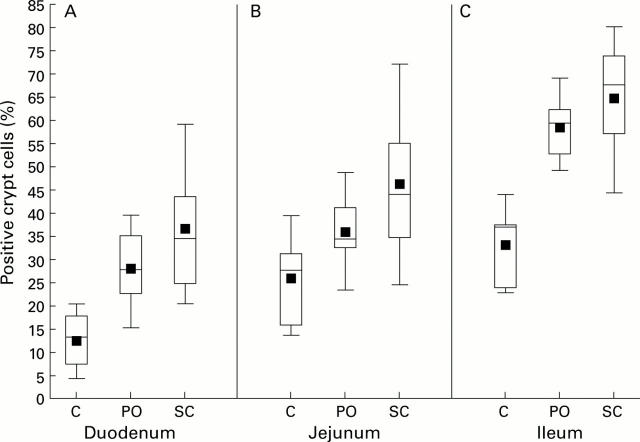
Figure 6 .
Bromodeoxyuridine (BrdU) positive crypt cells following no erythropoietin (Epo), enteral Epo, and parenteral Epo in the duodenum (A), jejunum (B), and ileum (C). Percentage of crypt cells with BrdU staining in each treatment group: control (no exogenous recombinant human erythropoietin (rEpo)), PO (dam received daily dose of 200 U rEpo intraperitoneally), and SC (pups received daily dose of 200 U/kg/day rEpo subcutaneously). Mean values and percentiles as described in fig 1.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anagnostou A., Liu Z., Steiner M., Chin K., Lee E. S., Kessimian N., Noguchi C. T. Erythropoietin receptor mRNA expression in human endothelial cells. Proc Natl Acad Sci U S A. 1994 Apr 26;91(9):3974–3978. doi: 10.1073/pnas.91.9.3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auestad N., Korsak R. A., Bergstrom J. D., Edmond J. Milk-substitutes comparable to rat's milk; their preparation, composition and impact on development and metabolism in the artificially reared rat. Br J Nutr. 1989 May;61(3):495–518. doi: 10.1079/bjn19890139. [DOI] [PubMed] [Google Scholar]
- Ballin A., Bilker-Reich A., Arbel E., Davidovitz Y., Kohelet D. Erythropoietin, given enterally, stimulates erythropoiesis in premature infants. Lancet. 1999 May 29;353(9167):1849–1849. doi: 10.1016/S0140-6736(99)01222-2. [DOI] [PubMed] [Google Scholar]
- Britton J. R., Christensen R. D. Enteral administration of recombinant erythropoietin to preterm infants. J Perinatol. 1995 Jul-Aug;15(4):281–283. [PubMed] [Google Scholar]
- Campana W. M., Misasi R., O'Brien J. S. Identification of a neurotrophic sequence in erythropoietin. Int J Mol Med. 1998 Jan;1(1):235–241. doi: 10.3892/ijmm.1.1.235. [DOI] [PubMed] [Google Scholar]
- Carmichael R. D., Gordon A. S., LoBue J. The effects of maternal phlebotomy and orally-administered erythropoietin (Ep) on erythropoiesis in the suckling rat. Biol Neonate. 1978;33(3-4):119–131. doi: 10.1159/000241061. [DOI] [PubMed] [Google Scholar]
- Carmichael R. D., Gordon A. S., Lobue J. Effects of the hormone erythropoietin in milk on erythropoiesis in neonatal rats. Endocrinol Exp. 1986 Aug;20(2-3):167–188. [PubMed] [Google Scholar]
- Carmichael R. D., LoBue J., Gordon A. S. Neonatal erythropoiesis. I. Peripheral blood erythropoietic parameters: data suggest erythropoietin transfer via maternal milk. Endocr Regul. 1992 Jun;26(2):83–88. [PubMed] [Google Scholar]
- Carmichael R. D., LoBue J., Gordon A. S. Neonatal erythropoiesis. II. Bone marrow and splenic erythropoietic activity: data suggest erythropoietin transfer via maternal milk. Endocr Regul. 1992 Sep;26(3):143–149. [PubMed] [Google Scholar]
- Carver J. D., Barness L. A. Trophic factors for the gastrointestinal tract. Clin Perinatol. 1996 Jun;23(2):265–285. [PubMed] [Google Scholar]
- Clapp J. F., 3rd, Little K. D., Appleby-Wineberg S. K., Widness J. A. The effect of regular maternal exercise on erythropoietin in cord blood and amniotic fluid. Am J Obstet Gynecol. 1995 May;172(5):1445–1451. doi: 10.1016/0002-9378(95)90476-x. [DOI] [PubMed] [Google Scholar]
- Fatouros M., Dalekos G. N., Mylonakis E., Vekinis G., Kappas A. M. Alterations in body weight, breaking strength, and wound healing in Wistar rats treated pre- and postoperatively with erythropoietin or granulocyte macrophage-colony stimulating factor: evidence of a previously unknown anabolic effect of erythropoietin? J Lab Clin Med. 1999 Mar;133(3):253–259. doi: 10.1016/s0022-2143(99)90081-1. [DOI] [PubMed] [Google Scholar]
- Hall W. G. Weaning and growth of artificially reared rats. Science. 1975 Dec 26;190(4221):1313–1315. doi: 10.1126/science.1198116. [DOI] [PubMed] [Google Scholar]
- Juul S. E., Joyce A. E., Zhao Y., Ledbetter D. J. Why is erythropoietin present in human milk? Studies of erythropoietin receptors on enterocytes of human and rat neonates. Pediatr Res. 1999 Sep;46(3):263–268. doi: 10.1203/00006450-199909000-00003. [DOI] [PubMed] [Google Scholar]
- Juul S. E., Yachnis A. T., Christensen R. D. Tissue distribution of erythropoietin and erythropoietin receptor in the developing human fetus. Early Hum Dev. 1998 Oct;52(3):235–249. doi: 10.1016/s0378-3782(98)00030-9. [DOI] [PubMed] [Google Scholar]
- Juul S. E., Zhao Y., Dame J. B., Du Y., Hutson A. D., Christensen R. D. Origin and fate of erythropoietin in human milk. Pediatr Res. 2000 Nov;48(5):660–667. doi: 10.1203/00006450-200011000-00018. [DOI] [PubMed] [Google Scholar]
- Kanto W. P., Jr, Hunter J. E., Stoll B. J. Recognition and medical management of necrotizing enterocolitis. Clin Perinatol. 1994 Jun;21(2):335–346. [PubMed] [Google Scholar]
- Kimble R. M., Breier B. H., Gluckman P. D., Harding J. E. Enteral IGF-I enhances fetal growth and gastrointestinal development in oesophageal ligated fetal sheep. J Endocrinol. 1999 Aug;162(2):227–235. doi: 10.1677/joe.0.1620227. [DOI] [PubMed] [Google Scholar]
- Kling P. J., Sullivan T. M., Roberts R. A., Philipps A. F., Koldovský O. Human milk as a potential enteral source of erythropoietin. Pediatr Res. 1998 Feb;43(2):216–221. doi: 10.1203/00006450-199802000-00010. [DOI] [PubMed] [Google Scholar]
- Ledbetter D. J., Juul S. E. Erythropoietin and the incidence of necrotizing enterocolitis in infants with very low birth weight. J Pediatr Surg. 2000 Feb;35(2):178–182. doi: 10.1016/s0022-3468(00)90006-x. [DOI] [PubMed] [Google Scholar]
- Marx C. E., Vance B. J., Jarskog L. F., Chescheir N. C., Gilmore J. H. Nerve growth factor, brain-derived neurotrophic factor, and neurotrophin-3 levels in human amniotic fluid. Am J Obstet Gynecol. 1999 Nov;181(5 Pt 1):1225–1230. doi: 10.1016/s0002-9378(99)70113-4. [DOI] [PubMed] [Google Scholar]
- Ohls R. K., Christensen R. D. Stability of human recombinant epoetin alfa in commonly used neonatal intravenous solutions. Ann Pharmacother. 1996 May;30(5):466–468. doi: 10.1177/106002809603000505. [DOI] [PubMed] [Google Scholar]
- Okada A., Kinoshita Y., Maekawa T., Hassan M. S., Kawanami C., Asahara M., Matsushima Y., Kishi K., Nakata H., Naribayashi Y. Erythropoietin stimulates proliferation of rat-cultured gastric mucosal cells. Digestion. 1996;57(5):328–332. doi: 10.1159/000201353. [DOI] [PubMed] [Google Scholar]
- Okamura M., Kurauchi O., Itakura A., Morikawa S., Suganuma N., Mizutani S., Tomoda Y. Hepatocyte growth factor in human amniotic fluid promotes the migration of fetal small intestinal epithelial cells. Am J Obstet Gynecol. 1998 Jan;178(1 Pt 1):175–179. doi: 10.1016/s0002-9378(98)70648-9. [DOI] [PubMed] [Google Scholar]
- Philipps A. F., Anderson G. G., Dvorák B., Williams C. S., Lake M., Lebouton A. V., Koldovský O. Growth of artificially fed infant rats: effect of supplementation with insulin-like growth factor I. Am J Physiol. 1997 May;272(5 Pt 2):R1532–R1539. doi: 10.1152/ajpregu.1997.272.5.R1532. [DOI] [PubMed] [Google Scholar]
- Richey S. D., Ramin S. M., Bawdon R. E., Roberts S. W., Dax J., Roberts J., Gilstrap L. C. Markers of acute and chronic asphyxia in infants with meconium-stained amniotic fluid. Am J Obstet Gynecol. 1995 Apr;172(4 Pt 1):1212–1215. doi: 10.1016/0002-9378(95)91481-1. [DOI] [PubMed] [Google Scholar]
- Rodgers C. T. Practical aspects of milk collection in the rat. Lab Anim. 1995 Oct;29(4):450–455. doi: 10.1258/002367795780739980. [DOI] [PubMed] [Google Scholar]
- Trahair J. F., Wing S. J., Quinn K. J., Owens P. C. Regulation of gastrointestinal growth in fetal sheep by luminally administered insulin-like growth factor-I. J Endocrinol. 1997 Jan;152(1):29–38. doi: 10.1677/joe.0.1520029. [DOI] [PubMed] [Google Scholar]
- Wen D., Boissel J. P., Tracy T. E., Gruninger R. H., Mulcahy L. S., Czelusniak J., Goodman M., Bunn H. F. Erythropoietin structure-function relationships: high degree of sequence homology among mammals. Blood. 1993 Sep 1;82(5):1507–1516. [PubMed] [Google Scholar]