Abstract
Arginine induced acute pancreatitis was evaluated as a novel and distinct form of experimental pancreatitis with particular attention to the actin cytoskeleton and expression of heat shock or stress proteins. Arginine induced a dose related necrotising pancreatitis in rats, as shown by histological evaluation, and an increase in serum amylase. Severe pancreatitis induced by 4.5 g/kg arginine was accompanied by dramatic changes in the actin cytoskeleton, as visualised with rhodamine phallodin. Intermediate filaments were also disrupted, as visualised by cytokeratin 8/18 immunocytochemistry. Arginine pancreatitis was accompanied by a stress response with a large increase in the small heat shock protein HSP27, as well as HSP70, peaking at 24 hours and localised to acinar cells. There was a lower increase in HSP60 and HSP90 and no effect on GRP78. HSP27 was also shifted to phosphorylated forms during pancreatitis. A lower dose of arginine (3.0 g/kg) induced less pancreatitis but a larger increase in HSP70 and HSP27 expression and phosphorylation of HSP27. Thus HSP expression can be overwhelmed by severe damage. The present work in conjunction with earlier work on caerulein induced pancreatitis indicates that changes in the actin cytoskeleton are an early component in experimental pancreatitis. Keywords: experimental pancreatitis; pancreas; actin cytoskeleton; cytokeratins; heat shock proteins; HSP27; rat
Full Text
The Full Text of this article is available as a PDF (439.2 KB).
Figure 1 .
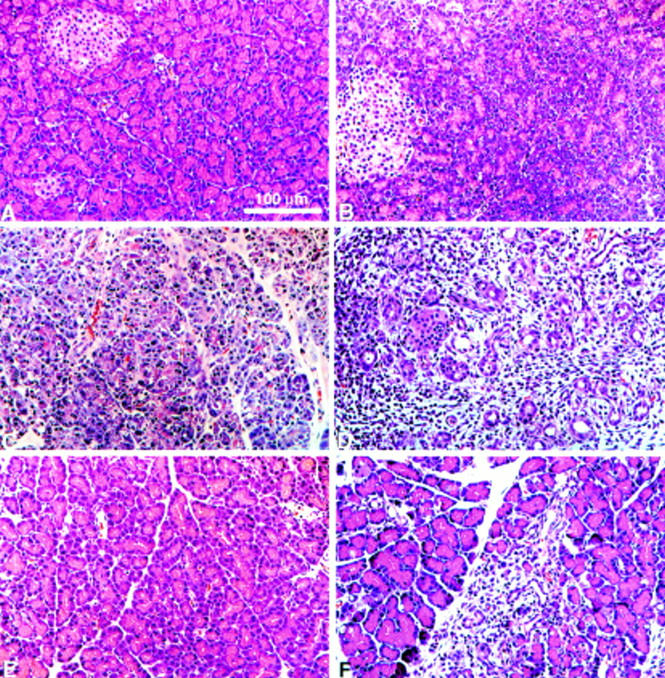
Light micrographs of the pancreas from control and arginine treated rats. Rats were injected with saline (A), 4.5 g/kg arginine (B, C, and D) or with 3.0 g/kg arginine (E and F). After injection with 4.5 g/kg arginine, the pancreas showed interstitial oedema, mild cellular infiltrate, and vacuoles within acinar cells at 12 hours (B), and by 24 hours acinar necrosis involved whole lobules and the acinar architecture was largely disrupted (C). After 72 hours (D), the only remaining acinar remnants were tubular complexes surrounded by fibroblasts and inflammatory cells. Injection of 3.0 g/kg arginine caused less severe pancreatic damage with very mild oedema after 24 hours (E). After 72 hours (F), most of the acinar cells appeared normal but there were small focal areas of fibrosis and inflammation. Haematoxylin and eosin staining.
Figure 2 .
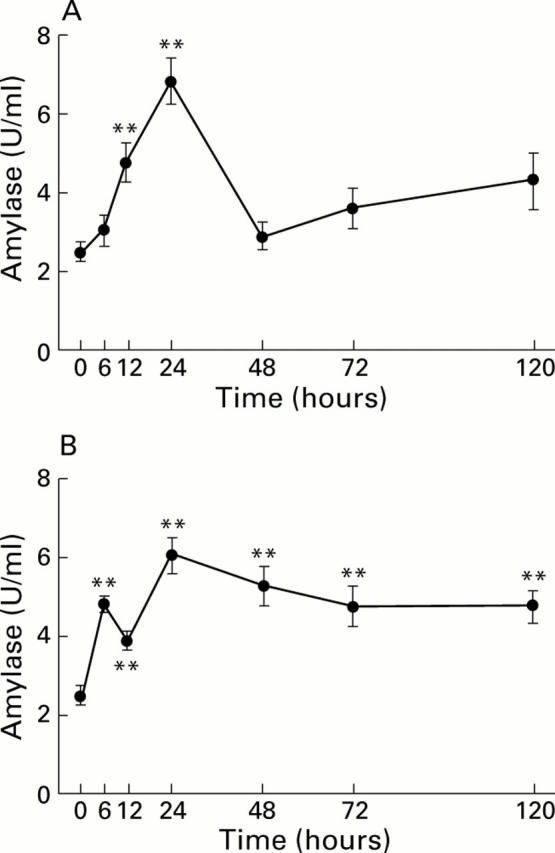
Time course of serum amylase levels in response to arginine induced acute pancreatitis. Results following administration of 4.5 g/kg arginine (A) or 3.0 g/kg arginine (B). Both show mean (SEM) values from 6-12 animals at each time point. **p<0.01 compared with control (0 hours).
Figure 3 .
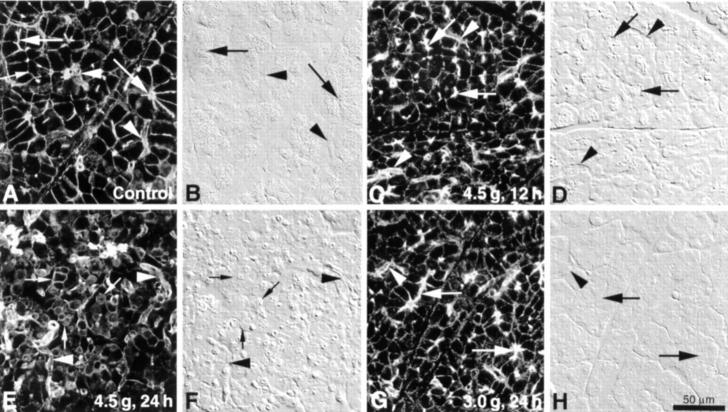
Effects of arginine injection on filamentous actin visualised with phalloidin in pancreatic acinar cells. Rats were injected with either 4.5 or 3.0 g/kg arginine, and pancreata were subsequently processed as described in material and methods. Paired Nomarski images were collected to provide bright field morphological detail. In control pancreatic acini (A, B), actin was present as sharply demarcated bands in the terminal web region just beneath the acinar lumen (large arrows) and subjacent to the lateral and basal plasma membranes. Actin was also present in duct and centroacinar cells (arrowheads). At 12 hours following injection with 4.5 g/kg arginine (C, D), moderate disorganisation/disruption of the normal actin staining pattern was apparent in many acini. Actin was less sharply defined along the basolateral membranes and often appeared discontinuous. Subluminal actin was present as in controls (large arrows) but the staining was often less sharply delineated. Acinar organisation appeared similar to controls (compare B and D). By 24 hours (E, F), the normal pattern of actin distribution was severely disrupted. Readily identifiable sub-luminal actin was no longer apparent, and acinar cells in poorly organised or fragmented acini were often rounded up and these cells generally expressed actin staining continuously along the cell periphery (small arrows in E). Diffuse cytoplasmic fluorescence was also present to varying degrees. Strong actin staining remained in the duct/centroacinar cells (arrowheads). In contrast, at 24 hours after injection with 3.0 g/kg arginine (G, H), acini retained their polarised state, and actin organisation was only moderately pertubed as was the case with the higher dose at 12 hours (compare C and G). Images are representative of at least four individual animals in each group.
Figure 4 .
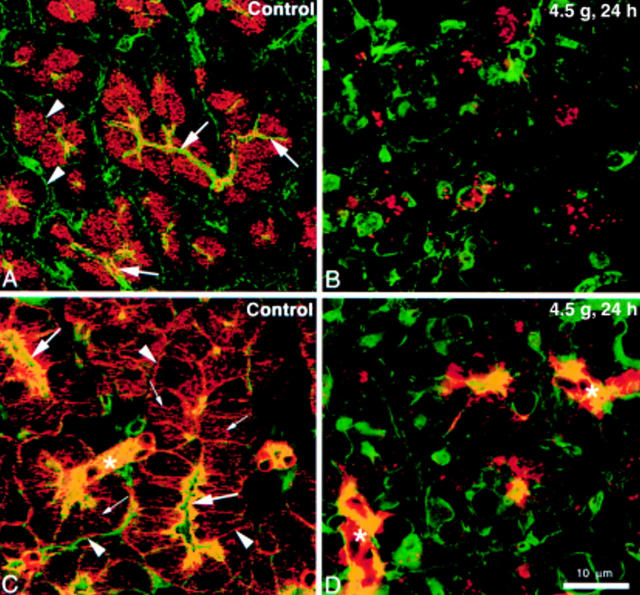
Effects of arginine injection on the distribution of amylase, cytokeratin, and actin. Cryostat sections from control animals (A, C) and in animals 24 hours post-injection (4.5 g/kg arginine) (B, D) were double labelled with BODIPY FL labelled phallacidin, and either antiamylase or anticytokeratin 8/18 antisera, followed by Cy3 conjugated second antibody. Immunofluorescence was monitored by confocal microscopy, and digitised images of FITC and Cy3 channels were superimposed. In control tissue (A), amylase (red fluorescence) is present in the secretory granules that fill the apical cytoplasm of acinar cells beneath the actin rich terminal web and lumen (arrows). Actin (green fluorescence) is also associated with basolateral membranes (arrowheads). Cytokeratin distribution (red fluorescence) in control acinar cells (C) is also present within the terminal web (large arrows) where it partially overlaps actin (yellow fluorescence) and along basolateral membranes (arrowheads). A thin band of cytokeratin free actin is present immediately beneath the luminal membrane (green fluorescence). In addition, filamentous cytokeratin strands extend in a rich network from the terminal region towards the basal aspects of the acinar cells (small arrows). Small ducts (asterisk) and their centroacinar cell termini express actin and abundant cytokeratin which overlap in their distribution. After 24 hours, the highly polarised distribution of actin, amylase, and cytokeratin in acinar cells is severely disrupted (B, D). Only a few small clusters of amylase containing secretory granules remain (B), and filamentous cytokeratin is lost, being replaced by a few focal deposits or small aggregates (D). Ducts and centroacinar cells (asterisk) retain actin and cytokeratin staining but in a markedly disorganised form. (A) and (B) are images reconstituted from complete Z series (1 µm steps) using Noran Intervision software. (C) and (D) are single optical sections taken at the focal plane in the Z series where both actin and cytokeratin filaments were defined most sharply.
Figure 5 .
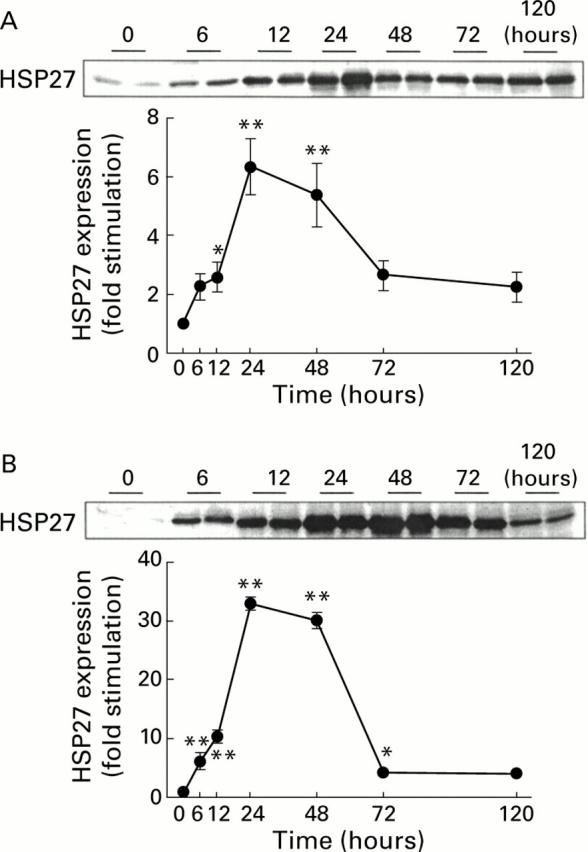
Time course of heat shock protein 27 (HSP27) expression in response to arginine induced acute pancreatitis. Representative western blots of pancreatic protein lysates from two rats following sodium dodecyl sulphate-polyacrylamide gel electrophoresis (30 µg/lane) are shown for each time point. Results following injection of 4.5 g/kg arginine (A) or 3.0 g/kg arginine (B). Both graphs show mean (SEM) values from 6-12 animals at each time point. In (A), the range of sample fold increases was six hours (0.6-4.6), 12 hours (0.3-5.1), 24 hours (2.5-10.7), 48 hours (1.0-9.0), 72 hours (0.5-6.5), and 120 hours (0.3-3.8). In (B), the range of fold increases was six hours (0.9-17.0), 12 hours (5.1-17.6), 24 hours (28.1-36.0), 48 hours (24.5-32.5), 72 hours (0.9-6.8), and 120 hours (2.0—6.4). *p<0.05; **p<0.01 compared with control (0 hours).
Figure 6 .
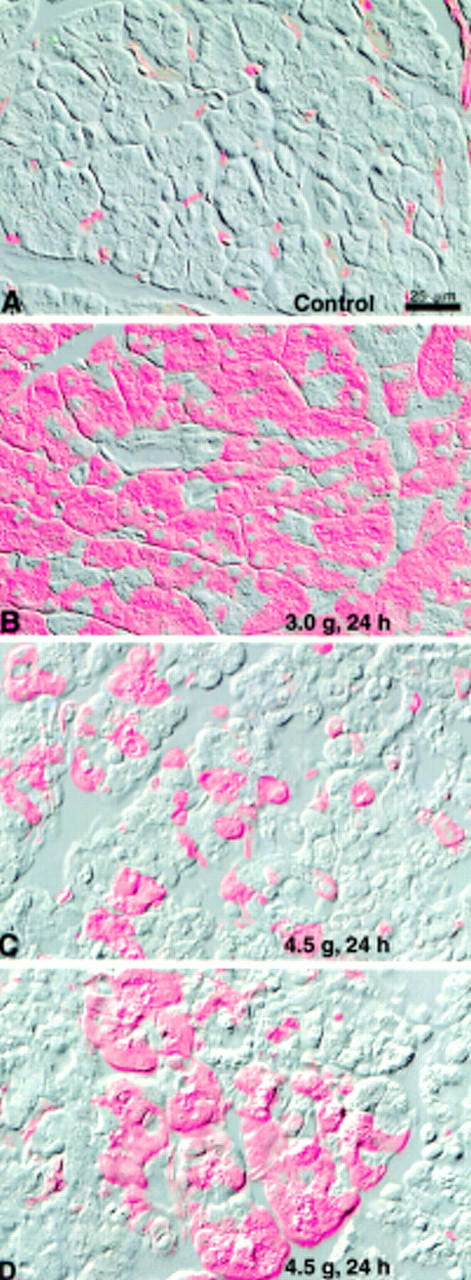
Localisation of heat shock protein 27 (HSP27) in the pancreas of control rats and 24 hours after 3.0 g/kg or 4.5 g/kg arginine injection. Epifluorescence images of HSP27 (coloured red) are overlaid on corresponding Nomarski images. In control rats (A), fluorescent HSP27 staining was limited to small blood vessels. After 3.0 g/kg arginine treatment (B), acinar cells were strongly labelled in a patchy manner. Ducts and blood vessels in the areas strongly expressing HSP27 in acinar cells showed little staining. After 4.5 g/kg arginine treatment (C, D), fewer acinar cells expressed HSP strongly whereas most showed weak or no expression. Acinar structure was compromised at the higher arginine dose, and oedematous areas were present (C). In some areas, foci of well organised acini were sometimes present (D). These acini expressed HSP27 whereas adjacent areas showed little HSP27 staining and poorly organised or disrupted acini. Images are representative of at least three individual animals in each group.
Figure 7 .
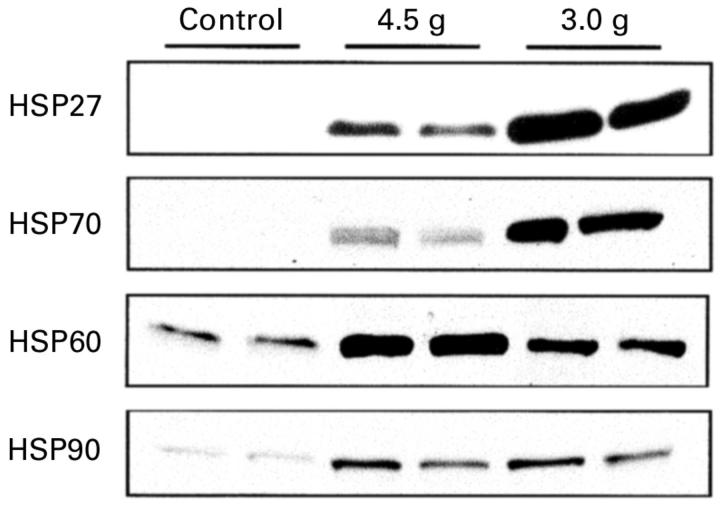
Analysis of heat shock proteins HSP27, HSP70, HSP60, and HSP90 expression at 24 hours after injection of 4.5 g/kg or 3.0 g/kg arginine or saline. Representative western blots of pancreatic protein lysates (30 µg/lane) from two rats following sodium dodecyl sulphate-polyacrylamide gel electrophoresis are shown for each dose of arginine (3.0 and 4.5 g).
Figure 8 .
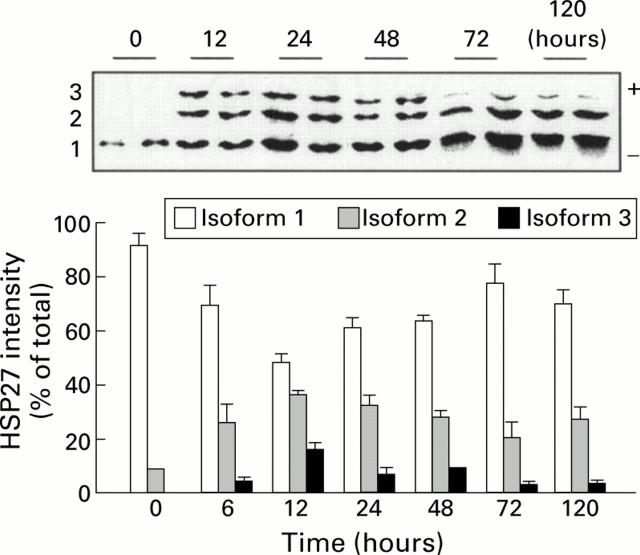
Time course of heat shock protein 27 (HSP27) protein phosphorylation in response to 4.5 g/kg arginine induced acute pancreatitis. Representative western blot analysis of pancreatic protein lysates from two rats following isoelectric focusing gel electrophoresis are shown for each time point. Isoform 1, unphosphorylated; isoform 2, monophosphorylated; and isoform 3, diphosphorylated. Both show mean (SEM) values for each isoform as a percentage of the total, obtained from 6-12 animals per time point.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler G., Kern H. F., Pan G. Z., Gardner J. D. Secretagogue-induced membrane alterations in dispersed acini from rat pancreas. Eur J Cell Biol. 1984 Mar;33(2):234–241. [PubMed] [Google Scholar]
- Aho H. J., Koskensalo S. M., Nevalainen T. J. Experimental pancreatitis in the rat. Sodium taurocholate-induced acute haemorrhagic pancreatitis. Scand J Gastroenterol. 1980;15(4):411–416. doi: 10.3109/00365528009181493. [DOI] [PubMed] [Google Scholar]
- Benndorf R., Hayess K., Ryazantsev S., Wieske M., Behlke J., Lutsch G. Phosphorylation and supramolecular organization of murine small heat shock protein HSP25 abolish its actin polymerization-inhibiting activity. J Biol Chem. 1994 Aug 12;269(32):20780–20784. [PubMed] [Google Scholar]
- Burnham D. B., Williams J. A. Effects of high concentrations of secretagogues on the morphology and secretory activity of the pancreas: a role for microfilaments. Cell Tissue Res. 1982;222(1):201–212. doi: 10.1007/BF00218300. [DOI] [PubMed] [Google Scholar]
- Czakó L., Takács T., Varga I. S., Tiszlavicz L., Hai D. Q., Hegyi P., Matkovics B., Lonovics J. Involvement of oxygen-derived free radicals in L-arginine-induced acute pancreatitis. Dig Dis Sci. 1998 Aug;43(8):1770–1777. doi: 10.1023/a:1018839821176. [DOI] [PubMed] [Google Scholar]
- Groblewski G. E., Grady T., Mehta N., Lambert H., Logsdon C. D., Landry J., Williams J. A. Cholecystokinin stimulates heat shock protein 27 phosphorylation in rat pancreas both in vivo and in vitro. Gastroenterology. 1997 Apr;112(4):1354–1361. doi: 10.1016/s0016-5085(97)70149-5. [DOI] [PubMed] [Google Scholar]
- Groblewski G. E., Yoshida M., Yao H., Williams J. A., Ernst S. A. Immunolocalization of CRHSP28 in exocrine digestive glands and gastrointestinal tissues of the rat. Am J Physiol. 1999 Jan;276(1 Pt 1):G219–G226. doi: 10.1152/ajpgi.1999.276.1.G219. [DOI] [PubMed] [Google Scholar]
- Hegyi P., Takács T., Jármay K., Nagy I., Czakó L., Lonovics J. Spontaneous and cholecystokinin-octapeptide-promoted regeneration of the pancreas following L-arginine-induced pancreatitis in rat. Int J Pancreatol. 1997 Dec;22(3):193–200. doi: 10.1007/BF02788384. [DOI] [PubMed] [Google Scholar]
- Huot J., Houle F., Marceau F., Landry J. Oxidative stress-induced actin reorganization mediated by the p38 mitogen-activated protein kinase/heat shock protein 27 pathway in vascular endothelial cells. Circ Res. 1997 Mar;80(3):383–392. doi: 10.1161/01.res.80.3.383. [DOI] [PubMed] [Google Scholar]
- Huot J., Houle F., Spitz D. R., Landry J. HSP27 phosphorylation-mediated resistance against actin fragmentation and cell death induced by oxidative stress. Cancer Res. 1996 Jan 15;56(2):273–279. [PubMed] [Google Scholar]
- Janmey P. A. The cytoskeleton and cell signaling: component localization and mechanical coupling. Physiol Rev. 1998 Jul;78(3):763–781. doi: 10.1152/physrev.1998.78.3.763. [DOI] [PubMed] [Google Scholar]
- Jungermann J., Lerch M. M., Weidenbach H., Lutz M. P., Krüger B., Adler G. Disassembly of rat pancreatic acinar cell cytoskeleton during supramaximal secretagogue stimulation. Am J Physiol. 1995 Feb;268(2 Pt 1):G328–G338. doi: 10.1152/ajpgi.1995.268.2.G328. [DOI] [PubMed] [Google Scholar]
- Kaufman R. J. Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational controls. Genes Dev. 1999 May 15;13(10):1211–1233. doi: 10.1101/gad.13.10.1211. [DOI] [PubMed] [Google Scholar]
- Kishino Y., Kawamura S. Pancreatic damage induced by injecting a large dose of arginine. Virchows Arch B Cell Pathol Incl Mol Pathol. 1984;47(2):147–155. doi: 10.1007/BF02890197. [DOI] [PubMed] [Google Scholar]
- Kishino Y., Takama S., Kitajima S. Ultracytochemistry of pancreatic damage induced by excess lysine. Virchows Arch B Cell Pathol Incl Mol Pathol. 1986;52(2):153–167. doi: 10.1007/BF02889959. [DOI] [PubMed] [Google Scholar]
- Klöppel G., Maillet B. Pathology of acute and chronic pancreatitis. Pancreas. 1993 Nov;8(6):659–670. doi: 10.1097/00006676-199311000-00001. [DOI] [PubMed] [Google Scholar]
- Ku N. O., Michie S., Oshima R. G., Omary M. B. Chronic hepatitis, hepatocyte fragility, and increased soluble phosphoglycokeratins in transgenic mice expressing a keratin 18 conserved arginine mutant. J Cell Biol. 1995 Dec;131(5):1303–1314. doi: 10.1083/jcb.131.5.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVY H. M., MONTANEZ G., FEAVER E. R., MURPHY E. A., DUNN M. S. Effect of arginine on tumor growth in rats. Cancer Res. 1954 Mar;14(3):198–200. [PubMed] [Google Scholar]
- Lampel M., Kern H. F. Acute interstitial pancreatitis in the rat induced by excessive doses of a pancreatic secretagogue. Virchows Arch A Pathol Anat Histol. 1977 Mar 11;373(2):97–117. doi: 10.1007/BF00432156. [DOI] [PubMed] [Google Scholar]
- Lavoie J. N., Gingras-Breton G., Tanguay R. M., Landry J. Induction of Chinese hamster HSP27 gene expression in mouse cells confers resistance to heat shock. HSP27 stabilization of the microfilament organization. J Biol Chem. 1993 Feb 15;268(5):3420–3429. [PubMed] [Google Scholar]
- Lerch M. M., Saluja A. K., Dawra R., Ramaraò P., Saluja M., Steer M. L. Acute necrotizing pancreatitis in the opossum: earliest morphological changes involve acinar cells. Gastroenterology. 1992 Jul;103(1):205–213. doi: 10.1016/0016-5085(92)91114-j. [DOI] [PubMed] [Google Scholar]
- Mehlen P., Kretz-Remy C., Préville X., Arrigo A. P. Human hsp27, Drosophila hsp27 and human alphaB-crystallin expression-mediated increase in glutathione is essential for the protective activity of these proteins against TNFalpha-induced cell death. EMBO J. 1996 Jun 3;15(11):2695–2706. [PMC free article] [PubMed] [Google Scholar]
- Milner J. A., Stepanovich L. V. Inhibitory effect of dietary arginine on growth of Ehrlich ascites tumor cells in mice. J Nutr. 1979 Mar;109(3):489–494. doi: 10.1093/jn/109.3.489. [DOI] [PubMed] [Google Scholar]
- Mizunuma T., Kawamura S., Kishino Y. Effects of injecting excess arginine on rat pancreas. J Nutr. 1984 Mar;114(3):467–471. doi: 10.1093/jn/114.3.467. [DOI] [PubMed] [Google Scholar]
- Morimoto R. I. Regulation of the heat shock transcriptional response: cross talk between a family of heat shock factors, molecular chaperones, and negative regulators. Genes Dev. 1998 Dec 15;12(24):3788–3796. doi: 10.1101/gad.12.24.3788. [DOI] [PubMed] [Google Scholar]
- Musch M. W., Sugi K., Straus D., Chang E. B. Heat-shock protein 72 protects against oxidant-induced injury of barrier function of human colonic epithelial Caco2/bbe cells. Gastroenterology. 1999 Jul;117(1):115–122. doi: 10.1016/s0016-5085(99)70557-3. [DOI] [PubMed] [Google Scholar]
- Nevalainen T. J., Seppä A. Acute pancreatitis caused by closed duodenal loop in the rat. Scand J Gastroenterol. 1975;10(5):521–527. [PubMed] [Google Scholar]
- O'Konski M. S., Pandol S. J. Effects of caerulein on the apical cytoskeleton of the pancreatic acinar cell. J Clin Invest. 1990 Nov;86(5):1649–1657. doi: 10.1172/JCI114887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnishi H., Samuelson L. C., Yule D. I., Ernst S. A., Williams J. A. Overexpression of Rab3D enhances regulated amylase secretion from pancreatic acini of transgenic mice. J Clin Invest. 1997 Dec 15;100(12):3044–3052. doi: 10.1172/JCI119859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohshio G., Saluja A., Steer M. L. Effects of short-term pancreatic duct obstruction in rats. Gastroenterology. 1991 Jan;100(1):196–202. doi: 10.1016/0016-5085(91)90601-g. [DOI] [PubMed] [Google Scholar]
- Otaka M., Okuyama A., Otani S., Jin M., Itoh S., Itoh H., Iwabuchi A., Sasahara H., Odashima M., Tashima Y. Differential induction of HSP60 and HSP72 by different stress situations in rats. Correlation with cerulein-induced pancreatitis. Dig Dis Sci. 1997 Jul;42(7):1473–1479. doi: 10.1023/a:1018866727129. [DOI] [PubMed] [Google Scholar]
- Piotrowicz R. S., Levin E. G. Basolateral membrane-associated 27-kDa heat shock protein and microfilament polymerization. J Biol Chem. 1997 Oct 10;272(41):25920–25927. doi: 10.1074/jbc.272.41.25920. [DOI] [PubMed] [Google Scholar]
- Sakagami J., Kataoka K., Ohta A., Nakajima T. Relationship of plasma CCK to acinar cell regeneration in acute pancreatitis as studied by proliferating cell nuclear antigen. Dig Dis Sci. 1996 Sep;41(9):1828–1837. doi: 10.1007/BF02088754. [DOI] [PubMed] [Google Scholar]
- Schäfer C., Clapp P., Welsh M. J., Benndorf R., Williams J. A. HSP27 expression regulates CCK-induced changes of the actin cytoskeleton in CHO-CCK-A cells. Am J Physiol. 1999 Dec;277(6 Pt 1):C1032–C1043. doi: 10.1152/ajpcell.1999.277.6.C1032. [DOI] [PubMed] [Google Scholar]
- Schäfer C., Ross S. E., Bragado M. J., Groblewski G. E., Ernst S. A., Williams J. A. A role for the p38 mitogen-activated protein kinase/Hsp 27 pathway in cholecystokinin-induced changes in the actin cytoskeleton in rat pancreatic acini. J Biol Chem. 1998 Sep 11;273(37):24173–24180. doi: 10.1074/jbc.273.37.24173. [DOI] [PubMed] [Google Scholar]
- Strowski M. Z., Sparmann G., Weber H., Fiedler F., Printz H., Jonas L., Göke B., Wagner A. C. Caerulein pancreatitis increases mRNA but reduces protein levels of rat pancreatic heat shock proteins. Am J Physiol. 1997 Oct;273(4 Pt 1):G937–G945. doi: 10.1152/ajpgi.1997.273.4.G937. [DOI] [PubMed] [Google Scholar]
- Tachibana I., Shirohara H., Czako L., Akiyama T., Nakano S., Watanabe N., Hirohata Y., Otsuki M. Role of endogenous cholecystokinin and cholecystokinin-A receptors in the development of acute pancreatitis in rats. Pancreas. 1997 Mar;14(2):113–121. doi: 10.1097/00006676-199703000-00002. [DOI] [PubMed] [Google Scholar]
- Takács T., Czakó L., Jármay K., Farkas G., Jr, Mándi Y., Lonovics J. Cytokine level changes in L-arginine-induced acute pancreatitis in rat. Acta Physiol Hung. 1996;84(2):147–156. [PubMed] [Google Scholar]
- Tani S., Itoh H., Okabayashi Y., Nakamura T., Fujii M., Fujisawa T., Koide M., Otsuki M. New model of acute necrotizing pancreatitis induced by excessive doses of arginine in rats. Dig Dis Sci. 1990 Mar;35(3):367–374. doi: 10.1007/BF01537416. [DOI] [PubMed] [Google Scholar]
- Toivola D. M., Ku N. O., Ghori N., Lowe A. W., Michie S. A., Omary M. B. Effects of keratin filament disruption on exocrine pancreas-stimulated secretion and susceptibility to injury. Exp Cell Res. 2000 Mar 15;255(2):156–170. doi: 10.1006/excr.1999.4787. [DOI] [PubMed] [Google Scholar]
- Torgerson R. R., McNiven M. A. The actin-myosin cytoskeleton mediates reversible agonist-induced membrane blebbing. J Cell Sci. 1998 Oct;111(Pt 19):2911–2922. doi: 10.1242/jcs.111.19.2911. [DOI] [PubMed] [Google Scholar]
- Varga I. S., Matkovics B., Czako L., Hai D. Q., Kotorman M., Takacs T., Sasvari M. Oxidative stress changes in L-arginine-induced pancreatitis in rats. Pancreas. 1997 May;14(4):355–359. doi: 10.1097/00006676-199705000-00005. [DOI] [PubMed] [Google Scholar]
- Wagner A. C., Weber H., Jonas L., Nizze H., Strowski M., Fiedler F., Printz H., Steffen H., Göke B. Hyperthermia induces heat shock protein expression and protection against cerulein-induced pancreatitis in rats. Gastroenterology. 1996 Nov;111(5):1333–1342. doi: 10.1053/gast.1996.v111.pm8898648. [DOI] [PubMed] [Google Scholar]
- Weber C. K., Gress T., Müller-Pillasch F., Lerch M. M., Weidenbach H., Adler G. Supramaximal secretagogue stimulation enhances heat shock protein expression in the rat pancreas. Pancreas. 1995 May;10(4):360–367. doi: 10.1097/00006676-199505000-00007. [DOI] [PubMed] [Google Scholar]