Abstract
BACKGROUND—Inflammatory bowel disease (IBD) is a chronic inflammation of the gastrointestinal tract caused by an abnormal and uncontrolled immune response to one or more normally occurring gut constituents. AIM—Given the effects of transforming growth factor β1 (TGF-β1) on both the immune system and extracellular matrix, we postulated that alterations in TGF-β signalling in intestinal epithelial cells may play an important role in the development of IBD. METHODS—TGF-β signalling was inactivated in mouse intestine by expressing a dominant negative mutant form of the TGF-β type II receptor under the control of the mouse intestinal trefoil peptide (ITF)/TFF3 promoter. Transgenic mice (ITF-dnRII) developed spontaneous colitis presenting with diarrhoea, haematochezia, and anal prolapse when not maintained under specific pathogen free (SPF) conditions. Under SPF conditions we induced colitis by mixing dextran sodium sulphate (DSS) in drinking water to examine the significance of loss of TGF-β signalling in the pathogenesis of IBD. RESULTS—Transgenic mice showed increased susceptibility to DSS induced IBD, and elicited increased expression of major histocompatibility complex class II, generation of autoantibodies against intestinal goblet cells, and increased activity of matrix metalloproteinase in intestinal epithelial cells compared with wild-type littermates challenged with DSS. CONCLUSIONS—Deficiency of TGF-β signalling specifically in the intestine contributes to the development of IBD. Maintenance of TGF-β signalling may be important in regulating immune homeostasis in the intestine Keywords: inflammatory bowel disease; transforming growth factor β; matrix metalloproteinases; intestinal trefoil factor; mouse
Full Text
The Full Text of this article is available as a PDF (492.8 KB).
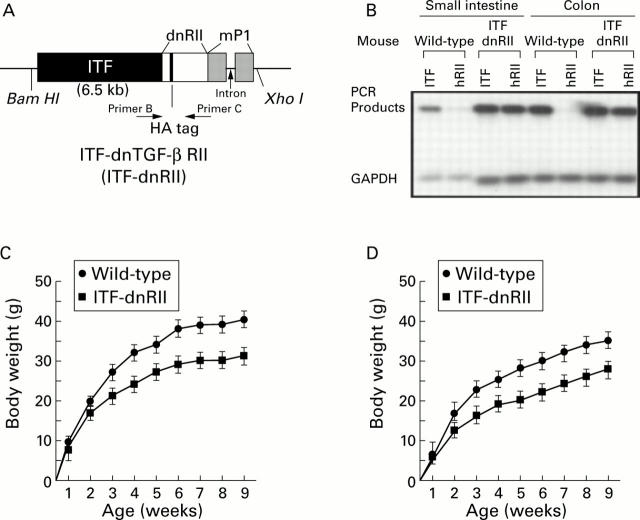
Figure 1 .
Generation of dominant negative mutant transforming growth factor β receptor II (TGF-β RII) mice—ITF-TGF-β dnRII transgenic mice. (A) Schematic representation of the transgene. The mouse intestinal trefoil factor (ITF) fragment spans −6500 bp to +35 bp. The 0.6 kb human TGF-β RII fragment spans +322 bp to + 911 bp and contains a haemagglutinin (HA) tag sequence and a segment of the mouse protamine that provides an intron and a polyadenylation site. Transgenic mice were generated using inbred FVB/N zygotes. Of the 15 mice born, six were positive for ITF-dnRII, bred into lines, and designated ITF1 to ITF6. Pups from ITF1, ITF2, and ITF3 were used for the current experiments. (B) TGF-β dnRII expression. Using total RNA from the intestines of wild-type mice and ITF-dnRII, reverse transcription-polymerase chain reaction was performed using the specific primer sets for human TGF-β RII, mouse ITF, and mouse GAPDH. (C, D) Body weights of wild-type littermates and ITF-dnRII transgenic mice. Body weight was measured every week for nine weeks in males (C) and females (D).
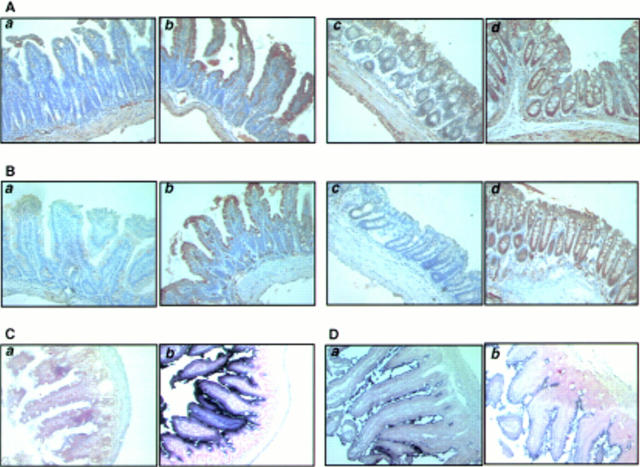
Figure 2 .
Tissue distribution of transforming growth factor β receptor II (TGF-β RII), intestinal trefoil factor (ITF), and dnRII. (A) Immunohistochemical staining of TGF-β RII using anti-TGF-β RII antibody and (B) TGF-β dnRII using anti-haemagglutinin (HA) tag antibody in the small intestine of wild-type littermates (a), small intestine of ITF-dnRII mice (b), colon of wild-type littermates (c), and colon of ITF-dnRII transgenic mice (d). Slightly increased expression of TGF-β type II was observed in the intestine of ITF-dnRII mice compared with wild-type littermates. Because we inserted the HA sequence in transgene construct, the HA tag was identified only in the intestine of ITF-dnRII mice by immunohistochemical staining with HA antibody; none was stained in the intestine of wild-type littermates. (C) In situ hybridisation was performed for the presence of human TGF-β RII mRNA using a biotinylated TGF-β RII probe (206 bp) in the colon of wild-type littermates (a) and the colon of ITF-dnRII transgenic mice (b). (D) In situ hybridisation of mouse ITF mRNA. Expression pattern of mouse ITF gene was similarly expressed in transgenic mice (a) and wild-type littermates (b) (×200 magnification)
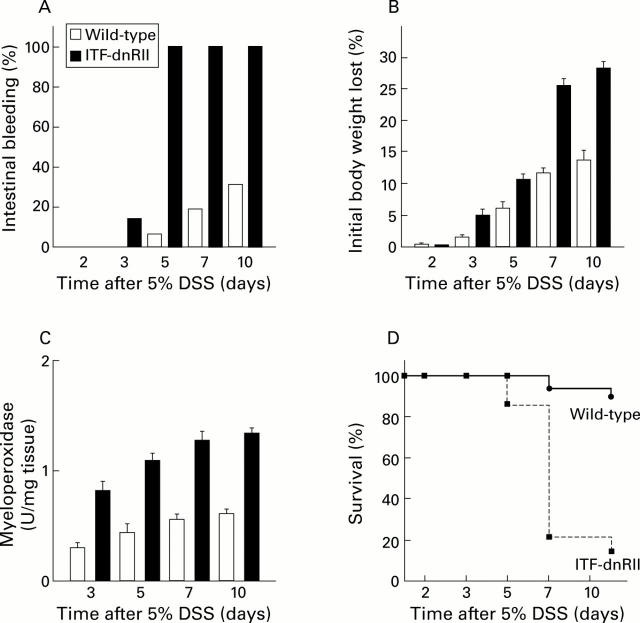
Figure 3 .
Dextran sodium sulphate (DSS) administration resulted in an increased incidence of intestinal bleeding, weight loss, colitis, and death in ITF-dnRII transgenic mice. (A) Prevalence of haematochezia. Intestinal bleeding (% incidence) was more common in ITF-dnRII mice. Earlier and more severe anal bleeding developed in mice lacking TGF-β signalling after administration of DSS (p<0.001). (B) Changes in body weight. Weight loss (expressed as per cent of initial body weight) was more prominent in ITF-dnRII mice than in wild-type mice. (C) Mucosal myeloperoxidase (MPO) activity. Changes in mucosal MPO activity in the colon of wild-type littermates and ITF-dnRII mice. ITF-dnRII mice showed significantly higher levels of mucosal MPO than wild-type mice (p<0.01). (D) Survival. Survival was significantly decreased in ITF-dnRII mice compared wild-type littermates using Kaplan-Meyer analysis. (ITF-dnRII transgenic mice were from ITF1-3 lines.)
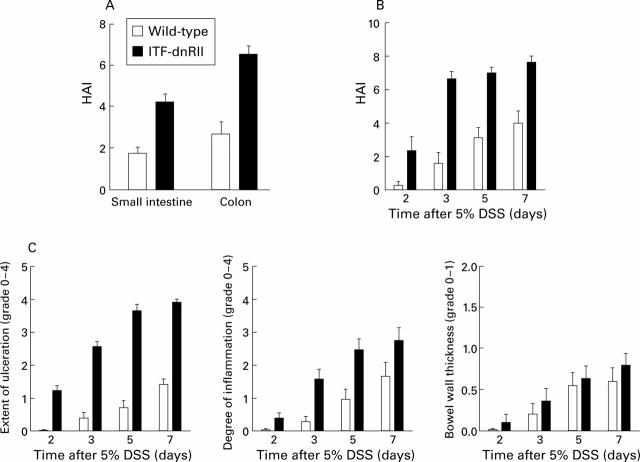
Figure 4 .
ITF-dnRII transgenic mice showed increased pathological scores Score criteria for extent of ulceration are as follows: 0, no architectural change; 1, focal superficial ulceration; 2, diffuse superficial ulceration; 3, focal gland dropout or focal deep ulceration; 4, extensive glandular dropout or deep ulceration. Scores for inflammation are as follows: 0, no increased inflammatory infiltrates; 1, focal mild inflammation; 2, diffuse mild inflammation; 3, cryptic abscess formation; 4, diffuse dense inflammation. Presence of oedema: 0, no significant oedema; 1, broad zone of oedema. Based on these criteria, comparison of histological activity index (HAI) is shown according to site of inflammation (A), days after 5% dextran sodium sulphate (DSS) administration (B), and criteria of each score (C). The extent of mucosal ulceration was significantly different between transgenic mice and wild-type littermates (p<0.001). (ITF-dnRII transgenic mice were from ITF1-3 lines.)
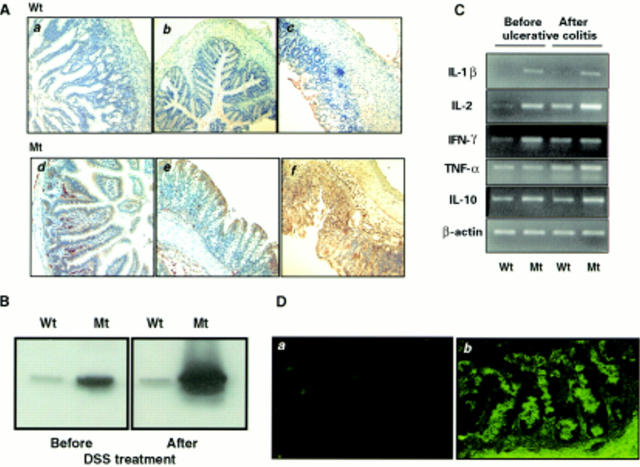
Figure 5 .
Increased expression of major histocompatibility complex (MHC) class II, cytokines, and autoantibodies in the intestine of ITF-dnRII transgenic mice. (A) Immunohistochemical staining of MHC class II. Scant MHC class II expression was observed in epithelial cells of the small intestine (a) and colon (b) of wild-type (Wt) FVB/N mice but increased expression of MHC class II was observed in epithelial cells of the small intestine (d) and colon (e) of ITF-dnRII transgenic mice (Mt). After induction of inflammatory bowel disease (IBD), MHC class II antigen expression was more markedly increased in transgenic mice (f) compared with wild-type mice (c) (×100 magnification). (B) Western blotting of MHC class II. Western blotting showed similar findings, with ITF-dnRII mice (Mt) showing increased expression of MHC class II antigens in intestine compared with wild-type littermates (Wt) (DSS, dextran sodium sulphate). (C) Changes in cytokines. Interleukin (IL)-1β, IL-2, interferon γ (IFN-γ), and IL-10 were all expressed at higher levels in the colons of transgenic mice (Mt) compared with those of wild-type mice (Wt). Expression of tumour necrosis factor α (TNF-α) was increased after induction of ulcerative colitis. (D) Autoantibodies in serum of ITF-dnRII transgenic mice. Autoantibodies were identified by indirect immunofluorescence using monkey ileum sections and FITC coupled rat antimouse immunoglobulin polyclonal antibody. Autoantibodies were detected in the serum of ITF-dnRII mice (b) whereas none was seen after applying sera obtained from wild-type littermates (a). Positive sera containing IgG autoantibodies showed blurry, drop-like staining (×200 magnification).
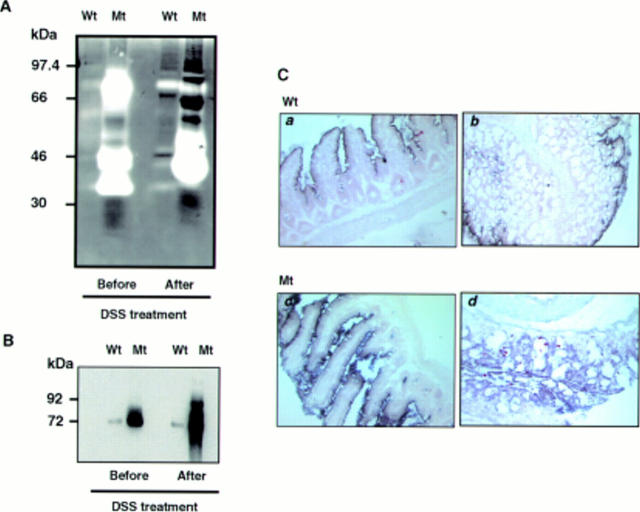
Figure 6 .
Matrix metalloproteinase (MMP) expression in mucosal homogenates of wild-type and transgenic mice. (A) Even before induction of ulcerative colitis, colon extracts of transgenic mice showed increased MMP gelatinolytic bands (52, 72, and 92 kDa) which become more prominent after induction of ulcerative colitis. Even applying only one tenth of the amount of protein compared with wild-type (Wt) littermates onto gelatin zymography gel, MMP activity was significantly increased in ITF-dnRII (Mt) mice. (B) MMP expression was confirmed by western blot analysis using a mixture of MMP-2, MMP-3, and MMP-9 antibodies. (C) In situ hybridisation of MMP-9 showed increased mRNA expression in small intestine (c) and colon (d) of ITF-dnRII transgenic mice (Mt) compared with wild-type littermates (Wt) (a, b) (×200 magnification).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BROBERGER O., PERLMANN P. Autoantibodies in human ulcerative colitis. J Exp Med. 1959 Nov 1;110:657–674. doi: 10.1084/jem.110.5.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babyatsky M. W., Rossiter G., Podolsky D. K. Expression of transforming growth factors alpha and beta in colonic mucosa in inflammatory bowel disease. Gastroenterology. 1996 Apr;110(4):975–984. doi: 10.1053/gast.1996.v110.pm8613031. [DOI] [PubMed] [Google Scholar]
- Bland P. W., Warren L. G. Antigen presentation by epithelial cells of the rat small intestine. II. Selective induction of suppressor T cells. Immunology. 1986 May;58(1):9–14. [PMC free article] [PubMed] [Google Scholar]
- Chinery R., Poulsom R., Cox H. M. The gene encoding mouse intestinal trefoil factor: structural organization, partial sequence analysis and mapping to murine chromosome 17q. Gene. 1996 Jun 1;171(2):249–253. doi: 10.1016/0378-1119(96)00074-1. [DOI] [PubMed] [Google Scholar]
- Cooper H. S., Murthy S. N., Shah R. S., Sedergran D. J. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993 Aug;69(2):238–249. [PubMed] [Google Scholar]
- Corcoran M. L., Hewitt R. E., Kleiner D. E., Jr, Stetler-Stevenson W. G. MMP-2: expression, activation and inhibition. Enzyme Protein. 1996;49(1-3):7–19. doi: 10.1159/000468613. [DOI] [PubMed] [Google Scholar]
- Delany A. M., Brinckerhoff C. E. Post-transcriptional regulation of collagenase and stromelysin gene expression by epidermal growth factor and dexamethasone in cultured human fibroblasts. J Cell Biochem. 1992 Dec;50(4):400–410. doi: 10.1002/jcb.240500409. [DOI] [PubMed] [Google Scholar]
- Edwards D. R., Murphy G., Reynolds J. J., Whitham S. E., Docherty A. J., Angel P., Heath J. K. Transforming growth factor beta modulates the expression of collagenase and metalloproteinase inhibitor. EMBO J. 1987 Jul;6(7):1899–1904. doi: 10.1002/j.1460-2075.1987.tb02449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger B., Procaccino F., Lakshmanan J., Reinshagen M., Hoffmann P., Patel A., Reuben W., Gnanakkan S., Liu L., Barajas L. Mice lacking transforming growth factor alpha have an increased susceptibility to dextran sulfate-induced colitis. Gastroenterology. 1997 Sep;113(3):825–832. doi: 10.1016/s0016-5085(97)70177-x. [DOI] [PubMed] [Google Scholar]
- Fiocchi C. Intestinal inflammation: a complex interplay of immune and nonimmune cell interactions. Am J Physiol. 1997 Oct;273(4 Pt 1):G769–G775. doi: 10.1152/ajpgi.1997.273.4.G769. [DOI] [PubMed] [Google Scholar]
- Flavell R. A., Allen H., Burkly L. C., Sherman D. H., Waneck G. L., Widera G. Molecular biology of the H-2 histocompatibility complex. Science. 1986 Jul 25;233(4762):437–443. doi: 10.1126/science.3726537. [DOI] [PubMed] [Google Scholar]
- Folwaczny C., Noehl N., Tschöp K., Endres S. P., Heldwein W., Loeschke K., Fricke H. Goblet cell autoantibodies in patients with inflammatory bowel disease and their first-degree relatives. Gastroenterology. 1997 Jul;113(1):101–106. doi: 10.1016/s0016-5085(97)70085-4. [DOI] [PubMed] [Google Scholar]
- Geiser A. G., Letterio J. J., Kulkarni A. B., Karlsson S., Roberts A. B., Sporn M. B. Transforming growth factor beta 1 (TGF-beta 1) controls expression of major histocompatibility genes in the postnatal mouse: aberrant histocompatibility antigen expression in the pathogenesis of the TGF-beta 1 null mouse phenotype. Proc Natl Acad Sci U S A. 1993 Nov 1;90(21):9944–9948. doi: 10.1073/pnas.90.21.9944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham M. F., Bryson G. R., Diegelmann R. F. Transforming growth factor beta 1 selectively augments collagen synthesis by human intestinal smooth muscle cells. Gastroenterology. 1990 Aug;99(2):447–453. doi: 10.1016/0016-5085(90)91028-5. [DOI] [PubMed] [Google Scholar]
- Hahm K. B., Im Y. H., Lee C., Parks W. T., Bang Y. J., Green J. E., Kim S. J. Loss of TGF-beta signaling contributes to autoimmune pancreatitis. J Clin Invest. 2000 Apr;105(8):1057–1065. doi: 10.1172/JCI8337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibi T., Ohara M., Toda K., Hara A., Ogata H., Iwao Y., Watanabe N., Watanabe M., Hamada Y., Kobayashi K. In vitro anticolon antibody production by mucosal or peripheral blood lymphocytes from patients with ulcerative colitis. Gut. 1990 Dec;31(12):1371–1376. doi: 10.1136/gut.31.12.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawisz J. E., Sharon P., Stenson W. F. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Assessment of inflammation in rat and hamster models. Gastroenterology. 1984 Dec;87(6):1344–1350. [PubMed] [Google Scholar]
- Kulkarni A. B., Huh C. G., Becker D., Geiser A., Lyght M., Flanders K. C., Roberts A. B., Sporn M. B., Ward J. M., Karlsson S. Transforming growth factor beta 1 null mutation in mice causes excessive inflammatory response and early death. Proc Natl Acad Sci U S A. 1993 Jan 15;90(2):770–774. doi: 10.1073/pnas.90.2.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn R., Löhler J., Rennick D., Rajewsky K., Müller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993 Oct 22;75(2):263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- Lin H. Y., Wang X. F., Ng-Eaton E., Weinberg R. A., Lodish H. F. Expression cloning of the TGF-beta type II receptor, a functional transmembrane serine/threonine kinase. Cell. 1992 Feb 21;68(4):775–785. doi: 10.1016/0092-8674(92)90152-3. [DOI] [PubMed] [Google Scholar]
- MacDermott R. P. Alterations of the mucosal immune system in inflammatory bowel disease. J Gastroenterol. 1996 Dec;31(6):907–916. doi: 10.1007/BF02358624. [DOI] [PubMed] [Google Scholar]
- Mizoguchi E., Mizoguchi A., Chiba C., Niles J. L., Bhan A. K. Antineutrophil cytoplasmic antibodies in T-cell receptor alpha-deficient mice with chronic colitis. Gastroenterology. 1997 Dec;113(6):1828–1835. doi: 10.1016/s0016-5085(97)70002-7. [DOI] [PubMed] [Google Scholar]
- Okayasu I., Hatakeyama S., Yamada M., Ohkusa T., Inagaki Y., Nakaya R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990 Mar;98(3):694–702. doi: 10.1016/0016-5085(90)90290-h. [DOI] [PubMed] [Google Scholar]
- Saarialho-Kere U. K., Vaalamo M., Puolakkainen P., Airola K., Parks W. C., Karjalainen-Lindsberg M. L. Enhanced expression of matrilysin, collagenase, and stromelysin-1 in gastrointestinal ulcers. Am J Pathol. 1996 Feb;148(2):519–526. [PMC free article] [PubMed] [Google Scholar]
- Sadlack B., Merz H., Schorle H., Schimpl A., Feller A. C., Horak I. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell. 1993 Oct 22;75(2):253–261. doi: 10.1016/0092-8674(93)80067-o. [DOI] [PubMed] [Google Scholar]
- Sartor R. B. Cytokines in intestinal inflammation: pathophysiological and clinical considerations. Gastroenterology. 1994 Feb;106(2):533–539. doi: 10.1016/0016-5085(94)90614-9. [DOI] [PubMed] [Google Scholar]
- Scott H., Solheim B. G., Brandtzaeg P., Thorsby E. HLA-DR-like antigens in the epithelium of the human small intestine. Scand J Immunol. 1980;12(1):77–82. doi: 10.1111/j.1365-3083.1980.tb00043.x. [DOI] [PubMed] [Google Scholar]
- Shull M. M., Ormsby I., Kier A. B., Pawlowski S., Diebold R. J., Yin M., Allen R., Sidman C., Proetzel G., Calvin D. Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature. 1992 Oct 22;359(6397):693–699. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza R. F., Garrigue-Antar L., Lei J., Yin J., Appel R., Vellucci V. F., Zou T. T., Zhou X., Wang S., Rhyu M. G. Alterations of transforming growth factor-beta 1 receptor type II occur in ulcerative colitis-associated carcinomas, sporadic colorectal neoplasms, and esophageal carcinomas, but not in gastric neoplasms. Hum Cell. 1996 Sep;9(3):229–236. [PubMed] [Google Scholar]
- Souza R. F., Lei J., Yin J., Appel R., Zou T. T., Zhou X., Wang S., Rhyu M. G., Cymes K., Chan O. A transforming growth factor beta 1 receptor type II mutation in ulcerative colitis-associated neoplasms. Gastroenterology. 1997 Jan;112(1):40–45. doi: 10.1016/s0016-5085(97)70217-8. [DOI] [PubMed] [Google Scholar]
- Stöcker W., Otte M., Ulrich S., Normann D., Finkbeiner H., Stöcker K., Jantschek G., Scriba P. C. Autoimmunity to pancreatic juice in Crohn's disease. Results of an autoantibody screening in patients with chronic inflammatory bowel disease. Scand J Gastroenterol Suppl. 1987;139:41–52. doi: 10.3109/00365528709089774. [DOI] [PubMed] [Google Scholar]
- Wiman K., Curman B., Forsum U., Klareskog L., Malmnäs-Tjernlund U., Rask L., Trägårdh L., Peterson P. A. Occurrence of Ia antigens on tissues on non-lymphoid origin. Nature. 1978 Dec 14;276(5689):711–713. doi: 10.1038/276711a0. [DOI] [PubMed] [Google Scholar]