Abstract
BACKGROUND—Lamina propria (LPLs) and intraepithelial (IELs) lymphocytes are markedly increased in coeliac mucosa, and are thought to play a crucial role in the generation of villous atrophy in coeliac disease (CD). However, the mechanisms by which they mediate the killing of enterocytes in this condition are still poorly characterised. AIM—We investigated Fas mediated cytotoxicity and apoptosis of both LPLs and IELs, isolated from 10 untreated coeliac patients, 10 coeliac patients on a gluten free diet, and 10 biopsied controls. METHODS—Fas and Fas ligand expression were assessed by flow cytometry and immunocytochemistry. Lymphocyte cytotoxicity against Fas expressing Jurkat cells was determined by the Jam test. The effect of the antagonist ZB4 anti-Fas antibody on apoptotic activity exerted by coeliac lymphocytes against enterocytes was analysed. Lymphocyte apoptosis was assessed by oligonucleosome ELISA. RESULTS—LPLs and IELs showed increased apoptotic activity and higher levels of Fas ligand expression in untreated CD compared with treated CD patients and controls. Enterocyte apoptosis observed after coculturing coeliac lymphocytes and enterocytes in the presence of ZB4 antibody was reduced. In active CD, LPLs manifested increased apoptosis whereas IELs showed decreased apoptosis. CONCLUSIONS—Our results support the involvement of the Fas/Fas ligand system in CD associated enterocyte apoptosis. Increased LPL apoptosis is likely to downregulate mucosal inflammation whereas decreased IEL apoptosis could be responsible for autoimmune and malignant complications of CD. Keywords: apoptosis; coeliac disease; cytotoxicity assay; Fas/Fas ligand system; intraepithelial lymphocytes; lamina propria lymphocytes
Full Text
The Full Text of this article is available as a PDF (168.4 KB).
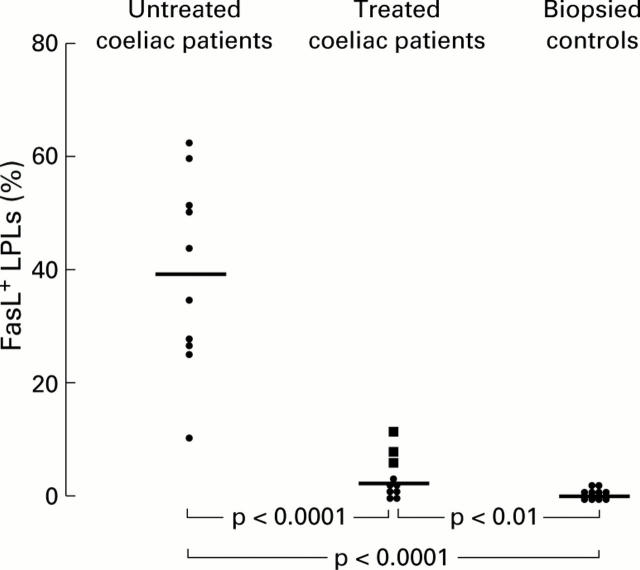
Figure 1 .
Percentages of FasL+ lamina propria lymphocytes (LPLs) in 10 untreated coeliac patients compared with those of 10 treated coeliac patients and 10 biopsied controls. Those coeliac patients on a gluten free diet and with a persistent degree of villous atrophy are indicated with a different symbol (solid square ). Horizontal bars represent median values.
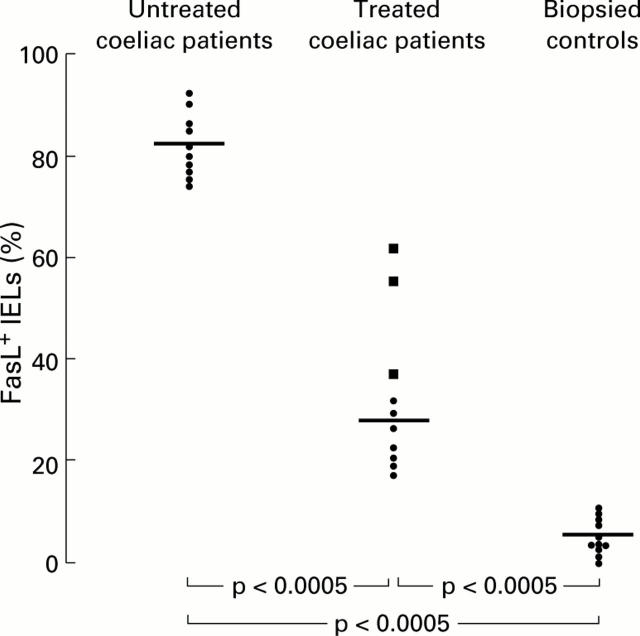
Figure 2 .
Percentages of FasL+ intraepithelial lymphocytes (IELs) in 10 untreated coeliac patients compared with those of 10 treated coeliac patients and 10 biopsied controls. Those coeliac patients on a gluten free diet and with a persistent degree of villous atrophy are indicated with a different symbol (solid square ). Horizontal bars represent median values.
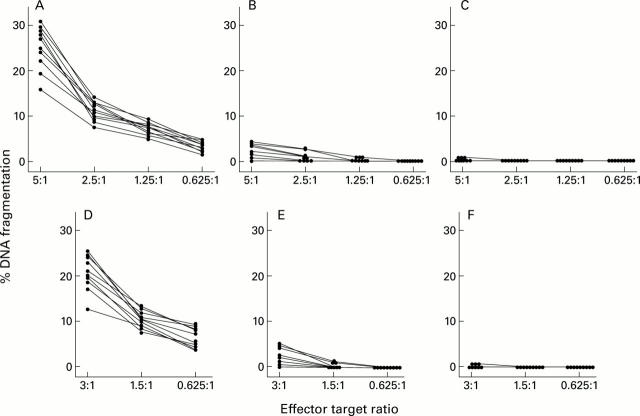
Figure 3 .
FasL mediated cytotoxicity by lamina propria lymphocytes (LPLs) (effectors) obtained from 10 untreated coeliac patients (A), 10 treated coeliac patients (B), and 10 biopsied controls (C) against Jurkat cells (targets). 3H labelled Jurkat cells were cultured with LPLs at various effector/target ratios (5:1; 2.5:1; 1.25:1; and 0.625:1). The percentage of DNA fragmentation of Jurkat cells was assayed after 12 hours. FasL mediated cytotoxicity by intraepithelial lymphocytes (IELs) (effectors) obtained from 10 untreated coeliac patients (D), 10 treated coeliac patients (E), and 10 biopsied controls (F) against Jurkat cells (targets). 3H labelled Jurkat cells were cultured with IELs at various effector/target ratios (3:1; 1.5:1; and 0.625:1). The percentage of DNA fragmentation of Jurkat cells was assayed after 12 hours.
Figure 4 .
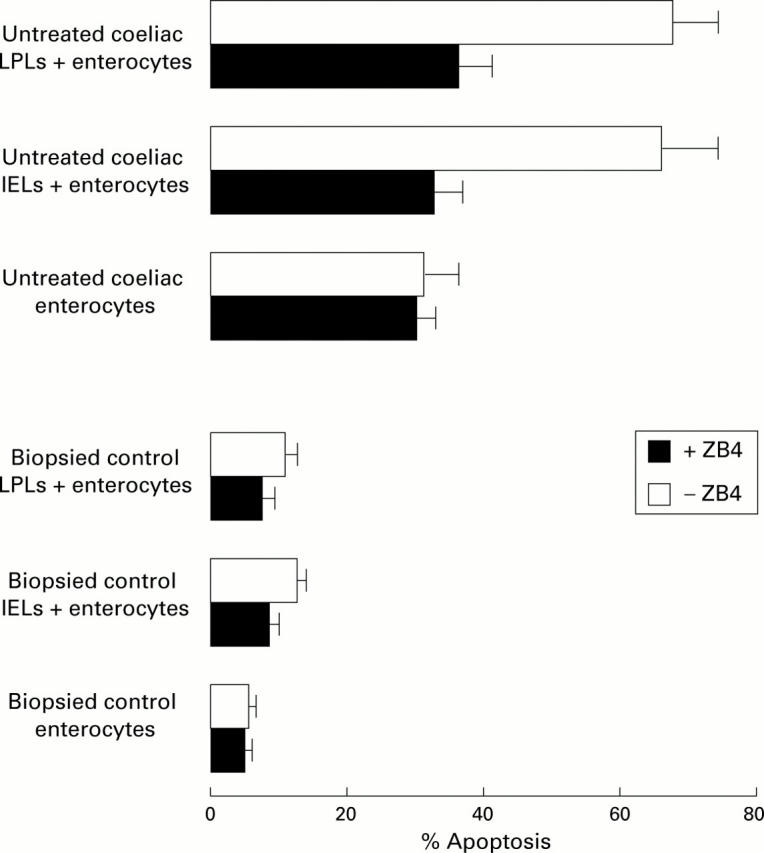
Antagonist ZB4 anti-Fas antibody reduces enterocyte apoptosis induced by both lamina propria lymphocyte (LPL) and intraepithelial lymphocyte (IEL) cytotoxic activity through a Fas/FasL interaction. Untreated coeliac and control enterocytes alone, and coculture of both untreated coeliac and control LPLs and IELs with enterocytes (at an effector target ratio of 20:1 and 100:1, respectively) were incubated for four hours in the presence or absence of 2 µg/ml ZB4 antagonist antibody. The percentage of apoptotic cells was determined by TUNEL detection.
Figure 5 .
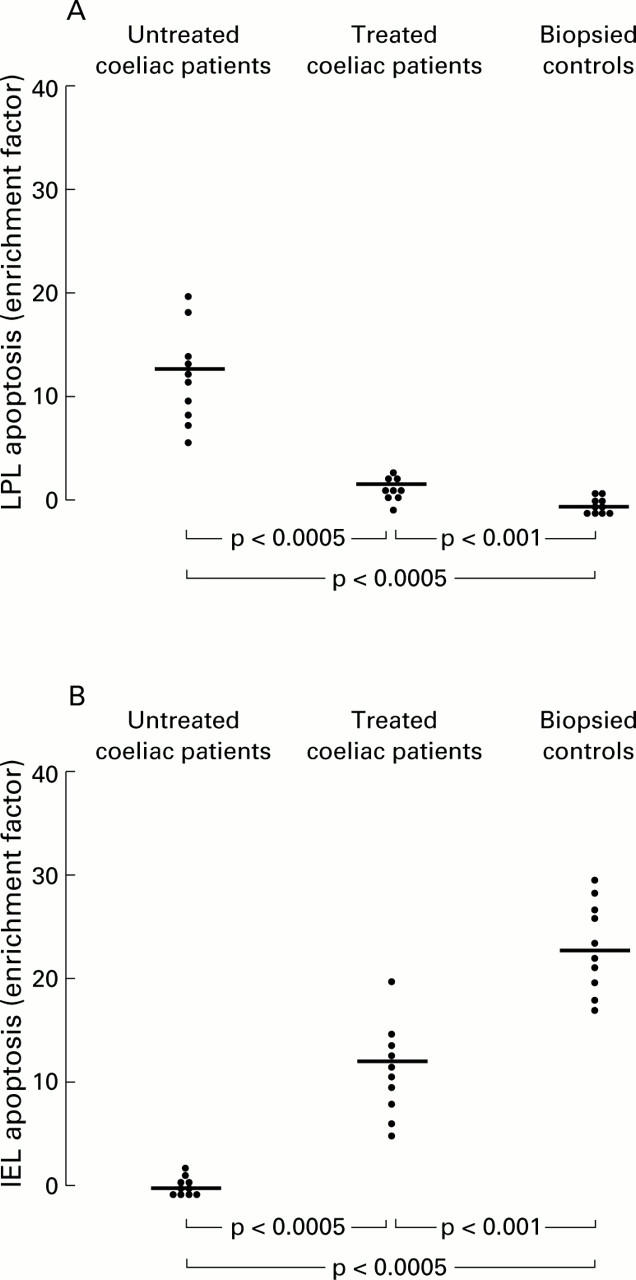
Detection of apoptosis on lamina propria lymphocytes (LPLs) (A) and intraepithelial lymphocytes (IELs) (B) by in vitro determination of cytoplasmic histone associated DNA fragments (mono and oligonucleosomes) in 10 untreated coeliac patients, 10 treated coeliac patients, and 10 biopsied controls. Quantitative results are expressed as an enrichment factor of mono and oligonucleosomes compared with controls. Horizontal bars represent median values.
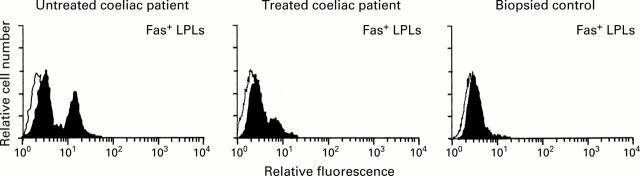
Figure 6 .
Representative flow cytometric histograms of Fas expression on lamina propria lymphocytes (LPLs) isolated from duodenal biopsy specimens of an untreated coeliac patient, a treated coeliac patient, and a biopsied control.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akbar A. N., Borthwick N., Salmon M., Gombert W., Bofill M., Shamsadeen N., Pilling D., Pett S., Grundy J. E., Janossy G. The significance of low bcl-2 expression by CD45RO T cells in normal individuals and patients with acute viral infections. The role of apoptosis in T cell memory. J Exp Med. 1993 Aug 1;178(2):427–438. doi: 10.1084/jem.178.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boirivant M., Pica R., DeMaria R., Testi R., Pallone F., Strober W. Stimulated human lamina propria T cells manifest enhanced Fas-mediated apoptosis. J Clin Invest. 1996 Dec 1;98(11):2616–2622. doi: 10.1172/JCI119082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulfone-Paus S., Ungureanu D., Pohl T., Lindner G., Paus R., Rückert R., Krause H., Kunzendorf U. Interleukin-15 protects from lethal apoptosis in vivo. Nat Med. 1997 Oct;3(10):1124–1128. doi: 10.1038/nm1097-1124. [DOI] [PubMed] [Google Scholar]
- Catassi C., Rossini M., Rätsch I. M., Bearzi I., Santinelli A., Castagnani R., Pisani E., Coppa G. V., Giorgi P. L. Dose dependent effects of protracted ingestion of small amounts of gliadin in coeliac disease children: a clinical and jejunal morphometric study. Gut. 1993 Nov;34(11):1515–1519. doi: 10.1136/gut.34.11.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cellier C., Patey N., Mauvieux L., Jabri B., Delabesse E., Cervoni J. P., Burtin M. L., Guy-Grand D., Bouhnik Y., Modigliani R. Abnormal intestinal intraepithelial lymphocytes in refractory sprue. Gastroenterology. 1998 Mar;114(3):471–481. doi: 10.1016/s0016-5085(98)70530-x. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R., Di Sabatino A., Parroni R., D'alò S., Pistoia M. A., Doglioni C., Cifone M. G., Corazza G. R. Cytolytic mechanisms of intraepithelial lymphocytes in coeliac disease (CoD). Clin Exp Immunol. 2000 May;120(2):235–240. doi: 10.1046/j.1365-2249.2000.01200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R., Di Sabatino A., Parroni R., Muzi P., D'Alò S., Ventura T., Pistoia M. A., Cifone M. G., Corazza G. R. Increased enterocyte apoptosis and Fas-Fas ligand system in celiac disease. Am J Clin Pathol. 2001 Apr;115(4):494–503. doi: 10.1309/UV54-BHP3-A66B-0QUD. [DOI] [PubMed] [Google Scholar]
- Corazza G. R., Frazzoni M., Dixon M. F., Gasbarrini G. Quantitative assessment of the mucosal architecture of jejunal biopsy specimens: a comparison between linear measurement, stereology, and computer aided microscopy. J Clin Pathol. 1985 Jul;38(7):765–770. doi: 10.1136/jcp.38.7.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daum S., Bauer U., Foss H. D., Schuppan D., Stein H., Riecken E. O., Ullrich R. Increased expression of mRNA for matrix metalloproteinases-1 and -3 and tissue inhibitor of metalloproteinases-1 in intestinal biopsy specimens from patients with coeliac disease. Gut. 1999 Jan;44(1):17–25. doi: 10.1136/gut.44.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhein J., Walczak H., Bäumler C., Debatin K. M., Krammer P. H. Autocrine T-cell suicide mediated by APO-1/(Fas/CD95) Nature. 1995 Feb 2;373(6513):438–441. doi: 10.1038/373438a0. [DOI] [PubMed] [Google Scholar]
- Dunnill M. S., Whitehead R. A method for the quantitation of small intestinal biopsy specimens. J Clin Pathol. 1972 Mar;25(3):243–246. doi: 10.1136/jcp.25.3.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert E. C. Do the CD45RO+CD8+ intestinal intraepithelial T lymphocytes have the characteristics of memory cells? Cell Immunol. 1993 Apr 1;147(2):331–340. doi: 10.1006/cimm.1993.1073. [DOI] [PubMed] [Google Scholar]
- Gelfanov V., Gelfanova V., Lai Y. G., Liao N. S. Activated alpha beta-CD8+, but not alpha alpha-CD8+, TCR-alpha beta+ murine intestinal intraepithelial lymphocytes can mediate perforin-based cytotoxicity, whereas both subsets are active in Fas-based cytotoxicity. J Immunol. 1996 Jan 1;156(1):35–41. [PubMed] [Google Scholar]
- Halstensen T. S., Brandtzaeg P. Activated T lymphocytes in the celiac lesion: non-proliferative activation (CD25) of CD4+ alpha/beta cells in the lamina propria but proliferation (Ki-67) of alpha/beta and gamma/delta cells in the epithelium. Eur J Immunol. 1993 Feb;23(2):505–510. doi: 10.1002/eji.1830230231. [DOI] [PubMed] [Google Scholar]
- Hershberg R. M., Mayer L. F. Antigen processing and presentation by intestinal epithelial cells - polarity and complexity. Immunol Today. 2000 Mar;21(3):123–128. doi: 10.1016/s0167-5699(99)01575-3. [DOI] [PubMed] [Google Scholar]
- Jabri B., de Serre N. P., Cellier C., Evans K., Gache C., Carvalho C., Mougenot J. F., Allez M., Jian R., Desreumaux P. Selective expansion of intraepithelial lymphocytes expressing the HLA-E-specific natural killer receptor CD94 in celiac disease. Gastroenterology. 2000 May;118(5):867–879. doi: 10.1016/S0016-5085(00)70173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janeway C. A., Jr, Jones B., Hayday A. Specificity and function of T cells bearing gamma delta receptors. Immunol Today. 1988 Mar;9(3):73–76. doi: 10.1016/0167-5699(88)91267-4. [DOI] [PubMed] [Google Scholar]
- Kabelitz D., Pohl T., Pechhold K. Activation-induced cell death (apoptosis) of mature peripheral T lymphocytes. Immunol Today. 1993 Jul;14(7):338–339. doi: 10.1016/0167-5699(93)90231-9. [DOI] [PubMed] [Google Scholar]
- Kawabata S., Boyaka P. N., Coste M., Fujihashi K., Yamamoto M., McGhee J. R., Kiyono H. Intraepithelial lymphocytes from villus tip and crypt portions of the murine small intestine show distinct characteristics. Gastroenterology. 1998 Oct;115(4):866–873. doi: 10.1016/s0016-5085(98)70258-6. [DOI] [PubMed] [Google Scholar]
- Kayagaki N., Kawasaki A., Ebata T., Ohmoto H., Ikeda S., Inoue S., Yoshino K., Okumura K., Yagita H. Metalloproteinase-mediated release of human Fas ligand. J Exp Med. 1995 Dec 1;182(6):1777–1783. doi: 10.1084/jem.182.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T., Brunner T., Tietz B., Madsen J., Bonfoco E., Reaves M., Huflejt M., Green D. R. Fas ligand- mediated killing by intestinal intraepithelial lymphocytes. Participation in intestinal graft-versus-host disease. J Clin Invest. 1998 Feb 1;101(3):570–577. doi: 10.1172/JCI896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald T. T., Spencer J. Evidence that activated mucosal T cells play a role in the pathogenesis of enteropathy in human small intestine. J Exp Med. 1988 Apr 1;167(4):1341–1349. doi: 10.1084/jem.167.4.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh M. N., Crowe P. T. Morphology of the mucosal lesion in gluten sensitivity. Baillieres Clin Gastroenterol. 1995 Jun;9(2):273–293. doi: 10.1016/0950-3528(95)90032-2. [DOI] [PubMed] [Google Scholar]
- Matzinger P. The JAM test. A simple assay for DNA fragmentation and cell death. J Immunol Methods. 1991 Dec 15;145(1-2):185–192. doi: 10.1016/0022-1759(91)90325-a. [DOI] [PubMed] [Google Scholar]
- Mayer M., Greco L., Troncone R., Auricchio S., Marsh M. N. Compliance of adolescents with coeliac disease with a gluten free diet. Gut. 1991 Aug;32(8):881–885. doi: 10.1136/gut.32.8.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretta A. Molecular mechanisms in cell-mediated cytotoxicity. Cell. 1997 Jul 11;90(1):13–18. doi: 10.1016/s0092-8674(00)80309-8. [DOI] [PubMed] [Google Scholar]
- Morimoto Y., Hizuta A., Ding E. X., Ishii T., Hongo T., Fujiwara T., Iwagaki H., Tanaka N. Functional expression of Fas and Fas ligand on human intestinal intraepithelial lymphocytes. Clin Exp Immunol. 1999 Apr;116(1):84–89. doi: 10.1046/j.1365-2249.1999.00827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss S. F., Attia L., Scholes J. V., Walters J. R., Holt P. R. Increased small intestinal apoptosis in coeliac disease. Gut. 1996 Dec;39(6):811–817. doi: 10.1136/gut.39.6.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsen E. M., Lundin K. E., Krajci P., Scott H., Sollid L. M., Brandtzaeg P. Gluten specific, HLA-DQ restricted T cells from coeliac mucosa produce cytokines with Th1 or Th0 profile dominated by interferon gamma. Gut. 1995 Dec;37(6):766–776. doi: 10.1136/gut.37.6.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishio A., Katakai T., Oshima C., Kasakura S., Sakai M., Yonehara S., Suda T., Nagata S., Masuda T. A possible involvement of Fas-Fas ligand signaling in the pathogenesis of murine autoimmune gastritis. Gastroenterology. 1996 Oct;111(4):959–967. doi: 10.1016/s0016-5085(96)70063-x. [DOI] [PubMed] [Google Scholar]
- Rocha B., von Boehmer H., Guy-Grand D. Selection of intraepithelial lymphocytes with CD8 alpha/alpha co-receptors by self-antigen in the murine gut. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5336–5340. doi: 10.1073/pnas.89.12.5336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai T., Kimura Y., Inagaki-Ohara K., Kusugami K., Lynch D. H., Yoshikai Y. Fas-mediated cytotoxicity by intestinal intraepithelial lymphocytes during acute graft-versus-host disease in mice. Gastroenterology. 1997 Jul;113(1):168–174. doi: 10.1016/s0016-5085(97)70092-1. [DOI] [PubMed] [Google Scholar]
- Simpson S. J., De Jong Y. P., Shah S. A., Comiskey M., Wang B., Spielman J. A., Podack E. R., Mizoguchi E., Bhan A. K., Terhorst C. Consequences of Fas-ligand and perforin expression by colon T cells in a mouse model of inflammatory bowel disease. Gastroenterology. 1998 Oct;115(4):849–855. doi: 10.1016/s0016-5085(98)70256-2. [DOI] [PubMed] [Google Scholar]
- Spencer J., Cerf-Bensussan N., Jarry A., Brousse N., Guy-Grand D., Krajewski A. S., Isaacson P. G. Enteropathy-associated T cell lymphoma (malignant histiocytosis of the intestine) is recognized by a monoclonal antibody (HML-1) that defines a membrane molecule on human mucosal lymphocytes. Am J Pathol. 1988 Jul;132(1):1–5. [PMC free article] [PubMed] [Google Scholar]
- Strasser A., Harris A. W., Huang D. C., Krammer P. H., Cory S. Bcl-2 and Fas/APO-1 regulate distinct pathways to lymphocyte apoptosis. EMBO J. 1995 Dec 15;14(24):6136–6147. doi: 10.1002/j.1460-2075.1995.tb00304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tew J. G., Phipps R. P., Mandel T. E. The maintenance and regulation of the humoral immune response: persisting antigen and the role of follicular antigen-binding dendritic cells as accessory cells. Immunol Rev. 1980;53:175–201. doi: 10.1111/j.1600-065x.1980.tb01044.x. [DOI] [PubMed] [Google Scholar]
- Ueyama H., Kiyohara T., Sawada N., Isozaki K., Kitamura S., Kondo S., Miyagawa J., Kanayama S., Shinomura Y., Ishikawa H. High Fas ligand expression on lymphocytes in lesions of ulcerative colitis. Gut. 1998 Jul;43(1):48–55. doi: 10.1136/gut.43.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M., Fujihashi K., Amano M., McGhee J. R., Beagley K. W., Kiyono H. Cytokine synthesis and apoptosis by intestinal intraepithelial lymphocytes: signaling of high density alpha beta T cell receptor+ and gamma delta T cell receptor+ T cells via T cell receptor-CD3 complex results in interferon-gamma and interleukin-5 production, while low density T cells undergo DNA fragmentation. Eur J Immunol. 1994 Jun;24(6):1301–1306. doi: 10.1002/eji.1830240609. [DOI] [PubMed] [Google Scholar]
- Yamamoto M., Fujihashi K., Kawabata K., McGhee J. R., Kiyono H. A mucosal intranet: intestinal epithelial cells down-regulate intraepithelial, but not peripheral, T lymphocytes. J Immunol. 1998 Mar 1;160(5):2188–2196. [PubMed] [Google Scholar]