Abstract
BACKGROUND—Epidemiological data indicate an increased risk for rectal cancer following chronic alcohol consumption. As chronic ethanol ingestion leads to rectal hyperregeneration in experimental animals, indicating a state of increased susceptibility to carcinogens, we studied cell proliferation in alcohol abusers. METHODS—Rectal biopsies were taken from 44 heavy drinkers and 26 controls. Cell proliferation, including proliferative compartment size, was measured by immunohistological staining for proliferative cell nuclear antigen (PCNA) and Ki67, and by in situ hybridisation for histone H3. Quantification of cell proliferation using PCNA staining was evaluated in 27 alcohol abusers and 12 controls. In addition, immunohistology was performed for cytokeratins and gene products of Rb1, bcl-2, and p53. RESULTS—Heavy drinking resulted in increased cell proliferation of the rectal mucosa, as shown by increased detection of different proliferation markers. However, cell differentiation regarding cytokeratin expression patterns was unchanged as well as regulatory factors involved in carcinogenesis and/or apoptosis. CONCLUSION—Chronic alcohol abuse leads to rectal mucosal hyperproliferation in humans, a condition associated with an increased cancer risk. Keywords: colorectal cancer; alcohol; rectal mucosa
Full Text
The Full Text of this article is available as a PDF (172.9 KB).
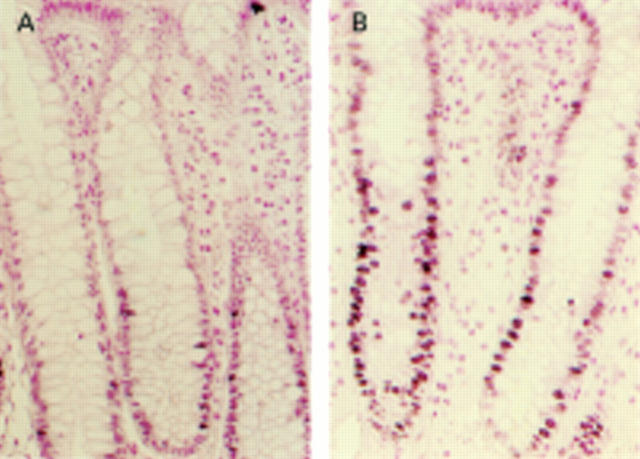
Figure 1 .
Typical staining patterns of proliferative cell nuclear antigen in nuclei of rectal crypts of controls (A) and alcohol abusers (B) (magnification ×100).
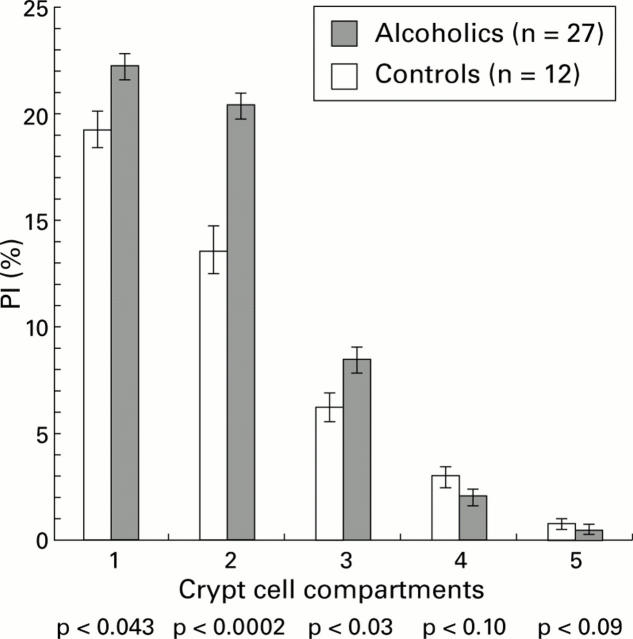
Figure 2 .
Proliferative indices (PI) in alcohol abusers and controls, expressed as a percentage of proliferative cell nuclear antigen positive cells in a rectal crypt, subdivided into equal crypt cell compartments, with compartment 1 being at the crypt base and compartment 5 adjacent to the crypt-luminar junction.
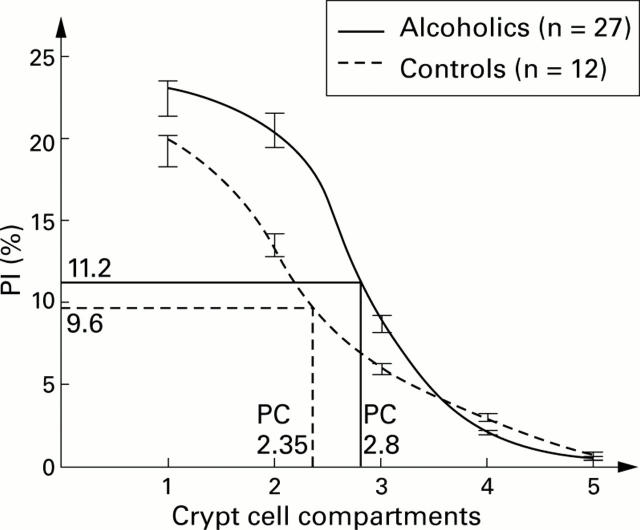
Figure 3 .
Graphically estimated proliferative compartment (PC) size, expressed as the number of crypt cell compartments, in alcohol abusers and controls. The limit of PC is 50% of the peak proliferative index (PI).
Figure 4 .
Staining for Ki67 in rectal crypts of controls (A) and alcohol abusers (B) (magnification ×100).
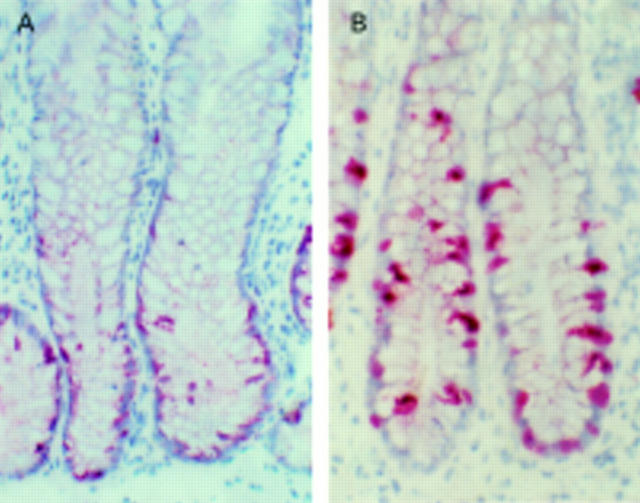
Figure 5 .
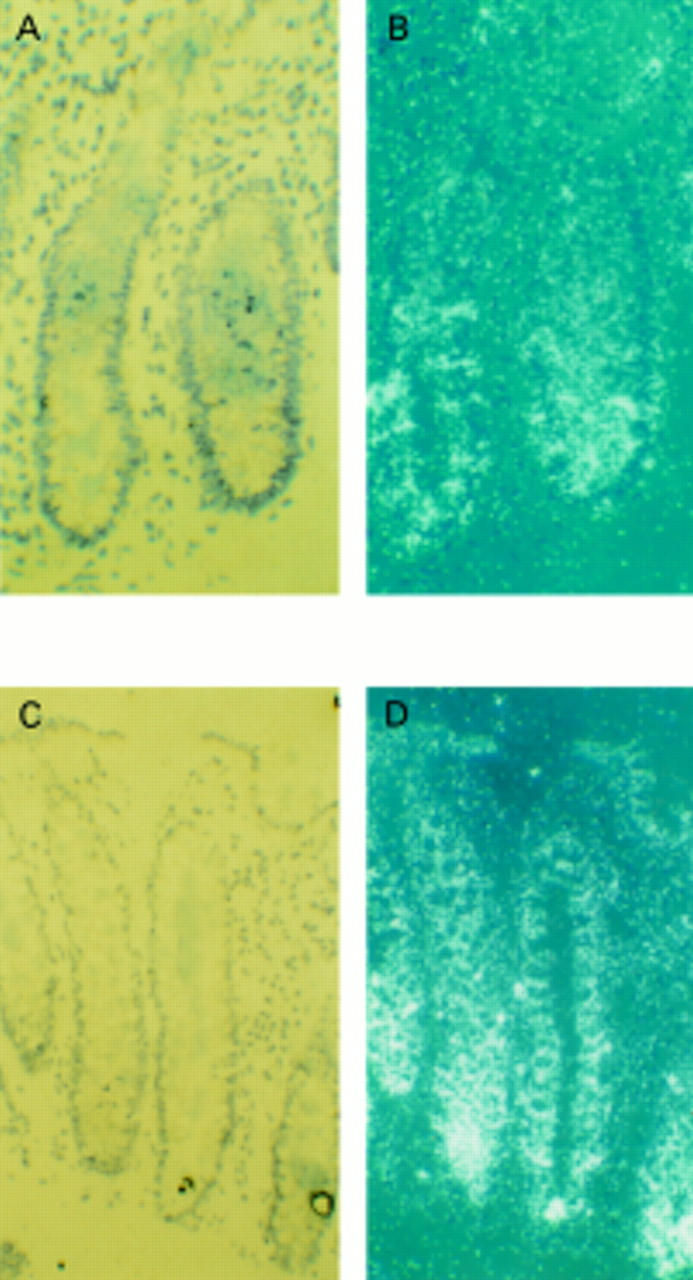
Histone H3 mRNA analysed by in situ hybridisation in rectal crypts of controls (A, B) and alcohol abusers (C, D). (B) and (D) are dark field photomicrographs of (A) and (C), respectively. There was considerably increased H3 mRNA in the proliferative regions of rectal crypts of alcoholic subjects (magnification ×80).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bosch F. X., Udvarhelyi N., Venter E., Herold-Mende C., Schuhmann A., Maier H., Weidauer H., Born A. I. Expression of the histone H3 gene in benign, semi-malignant and malignant lesions of the head and neck: a reliable proliferation marker. Eur J Cancer. 1993;29A(10):1454–1461. doi: 10.1016/0959-8049(93)90020-g. [DOI] [PubMed] [Google Scholar]
- Bosch F. X., Udvarhelyi N., Venter E., Herold-Mende C., Schuhmann A., Maier H., Weidauer H., Born A. I. Expression of the histone H3 gene in benign, semi-malignant and malignant lesions of the head and neck: a reliable proliferation marker. Eur J Cancer. 1993;29A(10):1454–1461. doi: 10.1016/0959-8049(93)90020-g. [DOI] [PubMed] [Google Scholar]
- Boutron-Ruault M. C., Senesse P., Faivre J., Couillault C., Belghiti C. Folate and alcohol intakes: related or independent roles in the adenoma-carcinoma sequence? Nutr Cancer. 1996;26(3):337–346. doi: 10.1080/01635589609514489. [DOI] [PubMed] [Google Scholar]
- Brozinsky S., Fani K., Grosberg S. J., Wapnick S. Alcohol ingestion-induced changes in the human rectal mucosa: light and electron microscopic studies. Dis Colon Rectum. 1978 Jul-Aug;21(5):329–335. doi: 10.1007/BF02586661. [DOI] [PubMed] [Google Scholar]
- Choi S. W., Stickel F., Baik H. W., Kim Y. I., Seitz H. K., Mason J. B. Chronic alcohol consumption induces genomic but not p53-specific DNA hypomethylation in rat colon. J Nutr. 1999 Nov;129(11):1945–1950. doi: 10.1093/jn/129.11.1945. [DOI] [PubMed] [Google Scholar]
- Deschner E. E., Lipkin M. Proliferative patterns in colonic mucosa in familial polyposis. Cancer. 1975 Feb;35(2):413–418. doi: 10.1002/1097-0142(197502)35:2<413::aid-cncr2820350217>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Deschner E. E., Long F. C., Hakissian M., Cupo S. H. Differential susceptibility of inbred mouse strains forecast by acute colonic proliferative response to methylazoxymethanol. J Natl Cancer Inst. 1984 Jan;72(1):195–198. doi: 10.1093/jnci/72.1.195. [DOI] [PubMed] [Google Scholar]
- Diehl A. M., Chacon M., Wagner P. The effect of chronic ethanol feeding on ornithine decarboxylase activity and liver regeneration. Hepatology. 1988 Mar-Apr;8(2):237–242. doi: 10.1002/hep.1840080208. [DOI] [PubMed] [Google Scholar]
- Eastwood G. L., Trier J. S. Epithelial cell renewal in cultured rectal biopsies in ulcerative colitis. Gastroenterology. 1973 Mar;64(3):383–390. [PubMed] [Google Scholar]
- Fearon E. R., Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990 Jun 1;61(5):759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- Giovannucci E., Rimm E. B., Ascherio A., Stampfer M. J., Colditz G. A., Willett W. C. Alcohol, low-methionine--low-folate diets, and risk of colon cancer in men. J Natl Cancer Inst. 1995 Feb 15;87(4):265–273. doi: 10.1093/jnci/87.4.265. [DOI] [PubMed] [Google Scholar]
- Hamilton S. R., Hyland J., McAvinchey D., Chaudhry Y., Hartka L., Kim H. T., Cichon P., Floyd J., Turjman N., Kessie G. Effects of chronic dietary beer and ethanol consumption on experimental colonic carcinogenesis by azoxymethane in rats. Cancer Res. 1987 Mar 15;47(6):1551–1559. [PubMed] [Google Scholar]
- Homann N., Kärkkäinen P., Koivisto T., Nosova T., Jokelainen K., Salaspuro M. Effects of acetaldehyde on cell regeneration and differentiation of the upper gastrointestinal tract mucosa. J Natl Cancer Inst. 1997 Nov 19;89(22):1692–1697. doi: 10.1093/jnci/89.22.1692. [DOI] [PubMed] [Google Scholar]
- Homann N., Tillonen J., Salaspuro M. Microbially produced acetaldehyde from ethanol may increase the risk of colon cancer via folate deficiency. Int J Cancer. 2000 Apr 15;86(2):169–173. doi: 10.1002/(sici)1097-0215(20000415)86:2<169::aid-ijc4>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Jokelainen K., Matysiak-Budnik T., Mäkisalo H., Höckerstedt K., Salaspuro M. High intracolonic acetaldehyde values produced by a bacteriocolonic pathway for ethanol oxidation in piglets. Gut. 1996 Jul;39(1):100–104. doi: 10.1136/gut.39.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jokelainen K., Nosova T., Koivisto T., Väkeväinen S., Jousimies-Somer H., Heine R., Salaspuro M. Inhibition of bacteriocolonic pathway for ethanol oxidation by ciprofloxacin in rats. Life Sci. 1997;61(18):1755–1762. doi: 10.1016/s0024-3205(97)00799-6. [DOI] [PubMed] [Google Scholar]
- Kearney J., Giovannucci E., Rimm E. B., Stampfer M. J., Colditz G. A., Ascherio A., Bleday R., Willett W. C. Diet, alcohol, and smoking and the occurrence of hyperplastic polyps of the colon and rectum (United States). Cancer Causes Control. 1995 Jan;6(1):45–56. doi: 10.1007/BF00051680. [DOI] [PubMed] [Google Scholar]
- Kikendall J. W., Bowen P. E., Burgess M. B., Magnetti C., Woodward J., Langenberg P. Cigarettes and alcohol as independent risk factors for colonic adenomas. Gastroenterology. 1989 Sep;97(3):660–664. doi: 10.1016/0016-5085(89)90637-9. [DOI] [PubMed] [Google Scholar]
- Kune G. A., Vitetta L. Alcohol consumption and the etiology of colorectal cancer: a review of the scientific evidence from 1957 to 1991. Nutr Cancer. 1992;18(2):97–111. doi: 10.1080/01635589209514210. [DOI] [PubMed] [Google Scholar]
- Lashner B. A., Heidenreich P. A., Su G. L., Kane S. V., Hanauer S. B. Effect of folate supplementation on the incidence of dysplasia and cancer in chronic ulcerative colitis. A case-control study. Gastroenterology. 1989 Aug;97(2):255–259. doi: 10.1016/0016-5085(89)90058-9. [DOI] [PubMed] [Google Scholar]
- Martínez M. E., McPherson R. S., Annegers J. F., Levin B. Cigarette smoking and alcohol consumption as risk factors for colorectal adenomatous polyps. J Natl Cancer Inst. 1995 Feb 15;87(4):274–279. doi: 10.1093/jnci/87.4.274. [DOI] [PubMed] [Google Scholar]
- Merritt A. J., Potten C. S., Watson A. J., Loh D. Y., Nakayama K., Nakayama K., Hickman J. A. Differential expression of bcl-2 in intestinal epithelia. Correlation with attenuation of apoptosis in colonic crypts and the incidence of colonic neoplasia. J Cell Sci. 1995 Jun;108(Pt 6):2261–2271. doi: 10.1242/jcs.108.6.2261. [DOI] [PubMed] [Google Scholar]
- Moll R., Franke W. W., Schiller D. L., Geiger B., Krepler R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell. 1982 Nov;31(1):11–24. doi: 10.1016/0092-8674(82)90400-7. [DOI] [PubMed] [Google Scholar]
- Pollack E. S., Nomura A. M., Heilbrun L. K., Stemmermann G. N., Green S. B. Prospective study of alcohol consumption and cancer. N Engl J Med. 1984 Mar 8;310(10):617–621. doi: 10.1056/NEJM198403083101003. [DOI] [PubMed] [Google Scholar]
- Reed J. C. Bcl-2 and the regulation of programmed cell death. J Cell Biol. 1994 Jan;124(1-2):1–6. doi: 10.1083/jcb.124.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg I. H., Mason J. B. Folate, dysplasia, and cancer. Gastroenterology. 1989 Aug;97(2):502–503. doi: 10.1016/0016-5085(89)90091-7. [DOI] [PubMed] [Google Scholar]
- Scheppach W., Bingham S., Boutron-Ruault M. C., Gerhardsson de Verdier M., Moreno V., Nagengast F. M., Reifen R., Riboli E., Seitz H. K., Wahrendorf J. WHO consensus statement on the role of nutrition in colorectal cancer. Eur J Cancer Prev. 1999 Feb;8(1):57–62. doi: 10.1097/00008469-199902000-00008. [DOI] [PubMed] [Google Scholar]
- Seitz H. K., Czygan P., Waldherr R., Veith S., Raedsch R., Kässmodel H., Kommerell B. Enhancement of 1,2-dimethylhydrazine-induced rectal carcinogenesis following chronic ethanol consumption in the rat. Gastroenterology. 1984 May;86(5 Pt 1):886–891. [PubMed] [Google Scholar]
- Seitz H. K., Egerer G., Oneta C., Krämer S., Sieg A., Klee F., Simanowski U. A. Alcohol dehydrogenase in the human colon and rectum. Digestion. 1996;57(2):105–108. doi: 10.1159/000201322. [DOI] [PubMed] [Google Scholar]
- Seitz H. K., Pöschl G., Simanowski U. A. Alcohol and cancer. Recent Dev Alcohol. 1998;14:67–95. doi: 10.1007/0-306-47148-5_4. [DOI] [PubMed] [Google Scholar]
- Seitz H. K., Simanowski U. A., Garzon F. T., Peters T. J. Alcohol and cancer. Hepatology. 1987 May-Jun;7(3):616–617. doi: 10.1002/hep.1840070349. [DOI] [PubMed] [Google Scholar]
- Seitz H. K., Simanowski U. A., Garzon F. T., Rideout J. M., Peters T. J., Koch A., Berger M. R., Einecke H., Maiwald M. Possible role of acetaldehyde in ethanol-related rectal cocarcinogenesis in the rat. Gastroenterology. 1990 Feb;98(2):406–413. doi: 10.1016/0016-5085(90)90832-l. [DOI] [PubMed] [Google Scholar]
- Shaw S., Jayatilleke E., Herbert V., Colman N. Cleavage of folates during ethanol metabolism. Role of acetaldehyde/xanthine oxidase-generated superoxide. Biochem J. 1989 Jan 1;257(1):277–280. doi: 10.1042/bj2570277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simanowski U. A., Seitz H. K., Baier B., Kommerell B., Schmidt-Gayk H., Wright N. A. Chronic ethanol consumption selectively stimulates rectal cell proliferation in the rat. Gut. 1986 Mar;27(3):278–282. doi: 10.1136/gut.27.3.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simanowski U. A., Suter P., Russell R. M., Heller M., Waldherr R., Ward R., Peters T. J., Smith D., Seitz H. K. Enhancement of ethanol induced rectal mucosal hyper regeneration with age in F344 rats. Gut. 1994 Aug;35(8):1102–1106. doi: 10.1136/gut.35.8.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaca C. E., Fang J. L., Schweda E. K. Studies of the reaction of acetaldehyde with deoxynucleosides. Chem Biol Interact. 1995 Oct 20;98(1):51–67. doi: 10.1016/0009-2797(95)03632-v. [DOI] [PubMed] [Google Scholar]
- Weisgerber U. M., Boeing H., Nemitz R., Raedsch R., Waldherr R. Proliferation cell nuclear antigen (clone 19A2) correlates with 5-bromo-2-deoxyuridine labelling in human colonic epithelium. Gut. 1993 Nov;34(11):1587–1592. doi: 10.1136/gut.34.11.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright N., Watson A., Morley A., Appleton D., Marks J. Cell kinetics in flat (avillous) mucosa of the human small intestine. Gut. 1973 Sep;14(9):701–710. doi: 10.1136/gut.14.9.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu A. H., Paganini-Hill A., Ross R. K., Henderson B. E. Alcohol, physical activity and other risk factors for colorectal cancer: a prospective study. Br J Cancer. 1987 Jun;55(6):687–694. doi: 10.1038/bjc.1987.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama A., Muramatsu T., Ohmori T., Yokoyama T., Okuyama K., Takahashi H., Hasegawa Y., Higuchi S., Maruyama K., Shirakura K. Alcohol-related cancers and aldehyde dehydrogenase-2 in Japanese alcoholics. Carcinogenesis. 1998 Aug;19(8):1383–1387. doi: 10.1093/carcin/19.8.1383. [DOI] [PubMed] [Google Scholar]
- Zhang M., Gong Y., Corbin I., Mellon A., Choy P., Uhanova J., Minuk G. Y. Light ethanol consumption enhances liver regeneration after partial hepatectomy in rats. Gastroenterology. 2000 Nov;119(5):1333–1339. doi: 10.1053/gast.2000.19281. [DOI] [PubMed] [Google Scholar]