Abstract
BACKGROUND AND AIMS—The benefit of 5-aminosalicylic acid therapy for maintenance of remission in Crohn's disease is controversial. The primary aim of this study was to evaluate the prophylactic properties of olsalazine in comparison with placebo for maintenance of remission in quiescent Crohn's colitis and/or ileocolitis. METHODS—In this randomised, double blind, parallel group study of olsalazine versus placebo, 328 patients with quiescent Crohn's colitis and/or ileocolitis were recruited. Treatment consisted of olsalazine 2.0 g daily or placebo for 52 weeks. The primary end point of efficacy was relapse, as defined by the Crohn's disease activity index (CDAI) and by clinical relapse. Laboratory and clinical disease activity indicators were also measured. Safety analysis consisted of documentation of adverse events and laboratory values. RESULTS—No differences in the frequency of termination due to relapse or time to termination due to relapse were noted between the two treatment groups (olsalazine 48.5% v placebo 45%) for either colitis or ileocolitis. The failure rate, defined as not completing the study, was significantly higher in olsalazine treated patients compared with placebo treated patients for the overall population (colitis and/or ileocolitis: olsalazine 65.4% v 53.9%; p=0.038). Similar failure rates were seen for patients with colitis. A significantly higher percentage of olsalazine treated patients experienced adverse gastrointestinal events. Drug attributed adverse events were reported more frequently in the olsalazine treated group with gastrointestinal symptoms being causally related to olsalazine treatment (olsalazine 40.7% v placebo 26.9%; p=0.010). Back pain was reported significantly more often by the placebo treated group. However, serious medical events did not differ between the two groups. Adverse events led to more early withdrawals in the olsalazine treated group than in the placebo treated group; thus average time in the study for patients in the olsalazine treatment group was significantly shorter than that of patients in the placebo group. CONCLUSIONS—Patients treated with olsalazine were more likely to terminate their participation in the trial than those taking placebo. This difference was not related to relapse of disease, as measured by CDAI and clinical measures, but rather was due to the development of intolerable adverse medical events of a non-serious nature related to the gastrointestinal tract. The gastrointestinal related events in the olsalazine treated group may be due to the difference in gastrointestinal status at baseline which favoured the placebo treatment group. Keywords: olsalazine; Crohn's disease; colitis; ileocolitis
Full Text
The Full Text of this article is available as a PDF (134.0 KB).
Figure 1 .
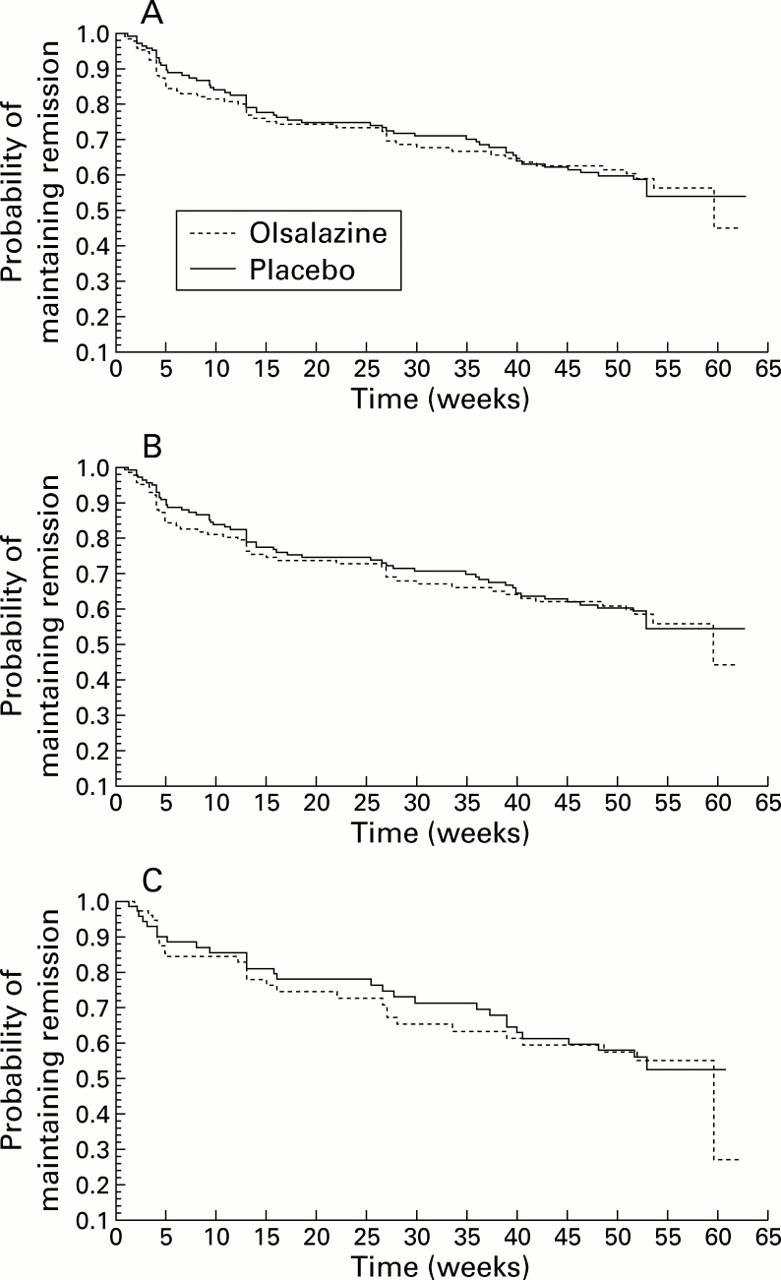
(A) Time to termination due to relapse in patients with Crohn's ileocolitis and/or colitis receiving maintenance therapy with olsalazine or placebo. (B) Time to termination due to relapse in patients with colonic Crohn's disease receiving maintenance therapy with olsalazine or placebo. (C) Time to termination due to relapse in patients with ileocaecal Crohn's disease receiving maintenance therapy with olsalazine or placebo.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arber N., Odes H. S., Fireman Z., Lavie A., Broide E., Bujanover Y., Becker S., Pomerantz I., Moshkowitz M., Patz J. A controlled double blind multicenter study of the effectiveness of 5-aminosalicylic acid in patients with Crohn's disease in remission. J Clin Gastroenterol. 1995 Apr;20(3):203–206. doi: 10.1097/00004836-199504000-00008. [DOI] [PubMed] [Google Scholar]
- Azad Khan A. K., Piris J., Truelove S. C. An experiment to determine the active therapeutic moiety of sulphasalazine. Lancet. 1977 Oct 29;2(8044):892–895. doi: 10.1016/s0140-6736(77)90831-5. [DOI] [PubMed] [Google Scholar]
- Best W. R., Becktel J. M., Singleton J. W., Kern F., Jr Development of a Crohn's disease activity index. National Cooperative Crohn's Disease Study. Gastroenterology. 1976 Mar;70(3):439–444. [PubMed] [Google Scholar]
- Cammà C., Giunta M., Rosselli M., Cottone M. Mesalamine in the maintenance treatment of Crohn's disease: a meta-analysis adjusted for confounding variables. Gastroenterology. 1997 Nov;113(5):1465–1473. doi: 10.1053/gast.1997.v113.pm9352848. [DOI] [PubMed] [Google Scholar]
- Dew M. J., Hughes P. J., Lee M. G., Evans B. K., Rhodes J. An oral preparation to release drugs in the human colon. Br J Clin Pharmacol. 1982 Sep;14(3):405–408. doi: 10.1111/j.1365-2125.1982.tb01999.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewe K., Herfarth C., Malchow H., Jesdinsky H. J. Postoperative recurrence of Crohn's disease in relation to radicality of operation and sulfasalazine prophylaxis: a multicenter trial. Digestion. 1989;42(4):224–232. doi: 10.1159/000199850. [DOI] [PubMed] [Google Scholar]
- Florent C., Cortot A., Quandale P., Sahmound T., Modigliani R., Sarfaty E., Valleur P., Dupas J. L., Daurat M., Faucheron J. L. Placebo-controlled clinical trial of mesalazine in the prevention of early endoscopic recurrences after resection for Crohn's disease. Groupe d'Etudes Thérapeutiques des Affections Inflammatoires Digestives (GETAID). Eur J Gastroenterol Hepatol. 1996 Mar;8(3):229–233. doi: 10.1097/00042737-199603000-00008. [DOI] [PubMed] [Google Scholar]
- Gendre J. P., Mary J. Y., Florent C., Modigliani R., Colombel J. F., Soulé J. C., Galmiche J. P., Lerebours E., Descos L., Viteau J. M. Oral mesalamine (Pentasa) as maintenance treatment in Crohn's disease: a multicenter placebo-controlled study. The Groupe d'Etudes Thérapeutiques des Affections Inflammatoires Digestives (GETAID) Gastroenterology. 1993 Feb;104(2):435–439. doi: 10.1016/0016-5085(93)90411-5. [DOI] [PubMed] [Google Scholar]
- Lochs H., Mayer M., Fleig W. E., Mortensen P. B., Bauer P., Genser D., Petritsch W., Raithel M., Hoffmann R., Gross V. Prophylaxis of postoperative relapse in Crohn's disease with mesalamine: European Cooperative Crohn's Disease Study VI. Gastroenterology. 2000 Feb;118(2):264–273. doi: 10.1016/s0016-5085(00)70208-3. [DOI] [PubMed] [Google Scholar]
- Modigliani R., Colombel J. F., Dupas J. L., Dapoigny M., Costil V., Veyrac M., Duclos B., Soulé J. C., Gendre J. P., Galmiche J. P. Mesalamine in Crohn's disease with steroid-induced remission: effect on steroid withdrawal and remission maintenance, Groupe d'Etudes Thérapeutiques des Affections Inflammatoires Digestives. Gastroenterology. 1996 Mar;110(3):688–693. doi: 10.1053/gast.1996.v110.pm8608877. [DOI] [PubMed] [Google Scholar]
- Myers B., Evans D. N., Rhodes J., Evans B. K., Hughes B. R., Lee M. G., Richens A., Richards D. Metabolism and urinary excretion of 5-amino salicylic acid in healthy volunteers when given intravenously or released for absorption at different sites in the gastrointestinal tract. Gut. 1987 Feb;28(2):196–200. doi: 10.1136/gut.28.2.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandberg-Gertzén H., Järnerot G., Kraaz W. Azodisal sodium in the treatment of ulcerative colitis. A study of tolerance and relapse-prevention properties. Gastroenterology. 1986 Apr;90(4):1024–1030. doi: 10.1016/0016-5085(86)90882-6. [DOI] [PubMed] [Google Scholar]
- Sandberg-Gertzén H., Ryde M., Järnerot G. Absorption and excretion of a single 1-g dose of azodisal sodium in subjects with ileostomy. Scand J Gastroenterol. 1983 Jan;18(1):107–111. doi: 10.3109/00365528309181568. [DOI] [PubMed] [Google Scholar]
- Selby W. S., Barr G. D., Ireland A., Mason C. H., Jewell D. P. Olsalazine in active ulcerative colitis. Br Med J (Clin Res Ed) 1985 Nov 16;291(6506):1373–1375. doi: 10.1136/bmj.291.6506.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland L. R., Martin F., Bailey R. J., Fedorak R. N., Poleski M., Dallaire C., Rossman R., Saibil F., Lariviere L. A randomized, placebo-controlled, double-blind trial of mesalamine in the maintenance of remission of Crohn's disease. The Canadian Mesalamine for Remission of Crohn's Disease Study Group. Gastroenterology. 1997 Apr;112(4):1069–1077. doi: 10.1016/s0016-5085(97)70117-3. [DOI] [PubMed] [Google Scholar]
- Taffet S. L., Das K. M. Sulfasalazine. Adverse effects and desensitization. Dig Dis Sci. 1983 Sep;28(9):833–842. doi: 10.1007/BF01296907. [DOI] [PubMed] [Google Scholar]
- Thomson A. B., Wright J. P., Vatn M., Bailey R. J., Rachmilewitz D., Adler M., Wilson-Lynch K. A. Mesalazine (Mesasal/Claversal) 1.5 g b.d. vs. placebo in the maintenance of remission of patients with Crohn's disease. Aliment Pharmacol Ther. 1995 Dec;9(6):673–683. doi: 10.1111/j.1365-2036.1995.tb00438.x. [DOI] [PubMed] [Google Scholar]
- Willoughby C. P., Aronson J. K., Agback H., Bodin N. O., Truelove S. C. Distribution and metabolism in healthy volunteers of disodium azodisalicylate, a potential therapeutic agent for ulcerative colitis. Gut. 1982 Dec;23(12):1081–1087. doi: 10.1136/gut.23.12.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hees P. A., Bakker J. H., van Tongeren J. H. Effect of sulphapyridine, 5-aminosalicylic acid, and placebo in patients with idiopathic proctitis: a study to determine the active therapeutic moiety of sulphasalazine. Gut. 1980 Jul;21(7):632–635. doi: 10.1136/gut.21.7.632. [DOI] [PMC free article] [PubMed] [Google Scholar]


