Abstract
BACKGROUND—It has been suggested that serological screening for coeliac disease (CD) should be performed in patients with chronic unexplained hypertransaminasaemia. AIMS—To evaluate the specificity for CD diagnosis of serum IgA antitissue transglutaminase (tTG) determination in consecutive patients with chronic hypertransaminasaemia using the most widely utilised ELISA based on tTG from guinea pig as the antigen. PATIENTS AND METHODS—We studied 98 patients with chronic hypertransaminasaemia, evaluated for the first time in a hepatology clinic. Serum anti-tTG and antiendomysial (EmA) assays were performed. Patients positive for EmA and/or anti-tTG were proposed for intestinal biopsy. Finally, all sera were reassayed for anti-tTG using an ELISA based on human recombinant tTG as the antigen. RESULTS—A total of 94/98 hypertransaminasaemic patients were positive for hepatitis virus markers, with 82/98 (83%) positive for anti-hepatitis C virus. Liver histology showed that most patients had mild or moderate chronic hepatitis while severe fibrosis or overt liver cirrhosis was found in 20/98. CD screening showed that 15/98 (16%) hypertransaminasaemic subjects had anti-tTG values in the same range as CD patients; however, IgA EmA were positive in only 2/98 (2%). Distal duodenal biopsy, performed in nine patients, showed subtotal villous atrophy in the two EmA+/anti-tTG+ patients but was normal in 7/7 EmA−/anti-tTG+ subjects. The presence of anti-tTG+ values in EmA− patients was unrelated to particular gastrointestinal symptoms, other associated diseases, severity of liver histology, or distribution of viral hepatitis markers. There was a significantly higher frequency of positive serum autoantibodies (antinuclear, antimitochondrial, antismooth muscle, and anti-liver-kidney microsomal antibodies) in anti-tTG+/EmA− patients than in the other subjects (9/13 v 10/83; p<0.003). Also, a correlation was found between serum gamma globulin and anti-tTG values (p<0.01). When sera were tested with the ELISA based on human tTG as the antigen, no false positive results were observed: only the two EmA+ patients with atrophy of the intestinal mucosa were positive for anti-tTG while all others were negative, including those false positive in the ELISA based on guinea pig tTG as the antigen. CONCLUSIONS—In patients with elevated transaminases and chronic liver disease there was a high frequency of false positive anti-tTG results using the ELISA based on tTG from guinea pig as the antigen. Indeed, the presence of anti-tTG did not correlate with the presence of EmA or CD. These false positives depend on the presence of hepatic proteins in the commercial tTG obtained from guinea pig liver and disappear when human tTG is used as the antigen in the ELISA system. We suggest that the commonly used tTG ELISA based on guinea pig antigen should not be used as a screening tool for CD in patients with chronic liver disease. Keywords: liver disease; coeliac disease; antitissue transglutaminase antibodies; antiendomysial antibodies; autoimmunity; intestinal histology
Full Text
The Full Text of this article is available as a PDF (185.2 KB).
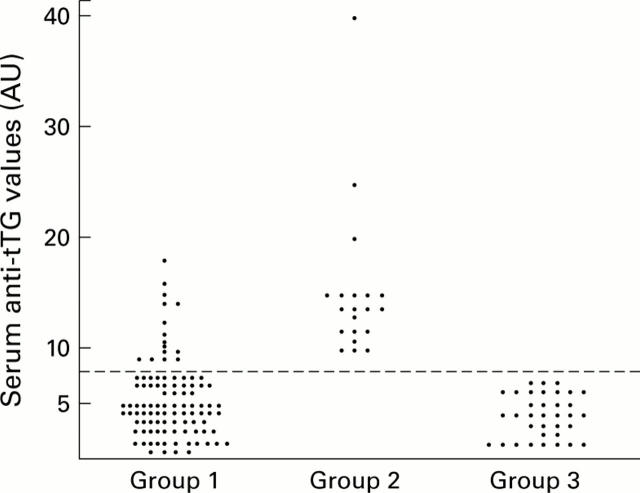
Figure 1 .
Serum IgA class tissue transglutaminase antibody (anti-tTG) titres determined by ELISA performed using Sigma tTG from guinea pig as the antigen. The results are expressed as AU values. The following groups were studied: group 1, hypertransaminasaemia patients (n=98); group 2, coeliac disease patients (EmA positive) on a gluten containing diet (n=20); group 3, healthy control subjects (EmA negative) (n=35). An arbitrary cut off level for positivity (broken line) was drawn at an AU of 8.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bardella M. T., Fraquelli M., Quatrini M., Molteni N., Bianchi P., Conte D. Prevalence of hypertransaminasemia in adult celiac patients and effect of gluten-free diet. Hepatology. 1995 Sep;22(3):833–836. [PubMed] [Google Scholar]
- Bardella M. T., Vecchi M., Conte D., Del Ninno E., Fraquelli M., Pacchetti S., Minola E., Landoni M., Cesana B. M., De Franchis R. Chronic unexplained hypertransaminasemia may be caused by occult celiac disease. Hepatology. 1999 Mar;29(3):654–657. doi: 10.1002/hep.510290318. [DOI] [PubMed] [Google Scholar]
- Bonamico M., Pitzalis G., Culasso F., Vania A., Monti S., Benedetti C., Mariani P., Signoretti A. Il danno epatico nella malattia celiaca del bambino. Minerva Pediatr. 1986 Nov 15;38(21):959–962. [PubMed] [Google Scholar]
- Brusco G., Izzi L., Corazza G. R. Tissue transglutaminase antibodies for coeliac disease screening. Ital J Gastroenterol Hepatol. 1998 Oct;30(5):496–497. [PubMed] [Google Scholar]
- Carroccio A., Cavataio F., Iacono G., Agate V., Ippolito S., Kazmierska I., Campagna P., Soresi M., Montalto G. IgA antiendomysial antibodies on the umbilical cord in diagnosing celiac disease. Sensitivity, specificity, and comparative evaluation with the traditional kit. Scand J Gastroenterol. 1996 Aug;31(8):759–763. doi: 10.3109/00365529609010348. [DOI] [PubMed] [Google Scholar]
- Carroccio A., Iacono G., Lerro P., Cavataio F., Malorgio E., Soresi M., Baldassarre M., Notarbartolo A., Ansaldi N., Montalto G. Role of pancreatic impairment in growth recovery during gluten-free diet in childhood celiac disease. Gastroenterology. 1997 Jun;112(6):1839–1844. doi: 10.1053/gast.1997.v112.pm9178674. [DOI] [PubMed] [Google Scholar]
- Carroccio A., Iannitto E., Cavataio F., Montalto G., Tumminello M., Campagna P., Lipari M. G., Notarbartolo A., Iacono G. Sideropenic anemia and celiac disease: one study, two points of view. Dig Dis Sci. 1998 Mar;43(3):673–678. doi: 10.1023/a:1018896015530. [DOI] [PubMed] [Google Scholar]
- Desmet V. J., Gerber M., Hoofnagle J. H., Manns M., Scheuer P. J. Classification of chronic hepatitis: diagnosis, grading and staging. Hepatology. 1994 Jun;19(6):1513–1520. [PubMed] [Google Scholar]
- Dieterich W., Ehnis T., Bauer M., Donner P., Volta U., Riecken E. O., Schuppan D. Identification of tissue transglutaminase as the autoantigen of celiac disease. Nat Med. 1997 Jul;3(7):797–801. doi: 10.1038/nm0797-797. [DOI] [PubMed] [Google Scholar]
- Dieterich W., Laag E., Schöpper H., Volta U., Ferguson A., Gillett H., Riecken E. O., Schuppan D. Autoantibodies to tissue transglutaminase as predictors of celiac disease. Gastroenterology. 1998 Dec;115(6):1317–1321. doi: 10.1016/s0016-5085(98)70007-1. [DOI] [PubMed] [Google Scholar]
- González-Abraldes J., Sánchez-Fueyo A., Bessa X., Moitinho E., Feu F., Mas A., Escorsell A., Bruguera M. Persistent hypertransaminasemia as the presenting feature of celiac disease. Am J Gastroenterol. 1999 Apr;94(4):1095–1097. doi: 10.1111/j.1572-0241.1999.01021.x. [DOI] [PubMed] [Google Scholar]
- Hagander B., Berg N. O., Brandt L., Nordén A., Sjölund K., Stenstam M. Hepatic injury in adult coeliac disease. Lancet. 1977 Aug 6;2(8032):270–272. doi: 10.1016/s0140-6736(77)90954-0. [DOI] [PubMed] [Google Scholar]
- Mäki M. Tissue transglutaminase as the autoantigen of coeliac disease. Gut. 1997 Oct;41(4):565–566. doi: 10.1136/gut.41.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera D. R., Weinstein W. M., Rubin C. E. Symposium on pathology of the gastrointestinal tract-Part II. Small intestinal biopsy. Hum Pathol. 1975 Mar;6(2):157–217. doi: 10.1016/s0046-8177(75)80176-6. [DOI] [PubMed] [Google Scholar]
- Revised criteria for diagnosis of coeliac disease. Report of Working Group of European Society of Paediatric Gastroenterology and Nutrition. Arch Dis Child. 1990 Aug;65(8):909–911. doi: 10.1136/adc.65.8.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sblattero D., Berti I., Trevisiol C., Marzari R., Tommasini A., Bradbury A., Fasano A., Ventura A., Not T. Human recombinant tissue transglutaminase ELISA: an innovative diagnostic assay for celiac disease. Am J Gastroenterol. 2000 May;95(5):1253–1257. doi: 10.1111/j.1572-0241.2000.02018.x. [DOI] [PubMed] [Google Scholar]
- Sjöberg K., Lindgren S., Eriksson S. Frequent occurrence of non-specific gliadin antibodies in chronic liver disease. Endomysial but not gliadin antibodies predict coeliac disease in patients with chronic liver disease. Scand J Gastroenterol. 1997 Nov;32(11):1162–1167. doi: 10.3109/00365529709002997. [DOI] [PubMed] [Google Scholar]
- Soresi M., Carroccio A., Bonfissuto G., Agate V., Magliarisi C., Aragona F., Levrero M., Notarbartolo A., Montalto G. Ultrasound detection of abdominal lymphadenomegaly in subjects with hepatitis C virus infection and persistently normal transaminases: a predictive index of liver histology severity. J Hepatol. 1998 Apr;28(4):544–549. doi: 10.1016/s0168-8278(98)80276-6. [DOI] [PubMed] [Google Scholar]
- Sulkanen S., Halttunen T., Laurila K., Kolho K. L., Korponay-Szabó I. R., Sarnesto A., Savilahti E., Collin P., Mäki M. Tissue transglutaminase autoantibody enzyme-linked immunosorbent assay in detecting celiac disease. Gastroenterology. 1998 Dec;115(6):1322–1328. doi: 10.1016/s0016-5085(98)70008-3. [DOI] [PubMed] [Google Scholar]
- Troncone R., Maurano F., Rossi M., Micillo M., Greco L., Auricchio R., Salerno G., Salvatore F., Sacchetti L. IgA antibodies to tissue transglutaminase: An effective diagnostic test for celiac disease. J Pediatr. 1999 Feb;134(2):166–171. doi: 10.1016/s0022-3476(99)70410-5. [DOI] [PubMed] [Google Scholar]
- Vajro P., Fontanella A., Mayer M., De Vincenzo A., Terracciano L. M., D'Armiento M., Vecchione R. Elevated serum aminotransferase activity as an early manifestation of gluten-sensitive enteropathy. J Pediatr. 1993 Mar;122(3):416–419. doi: 10.1016/s0022-3476(05)83430-4. [DOI] [PubMed] [Google Scholar]