Abstract
BACKGROUND—Invasion of the intestinal mucosa by leucocytes is a characteristic of intestinal inflammation but the role of the epithelium in orchestrating this recruitment has not been examined in vivo. Cultured intestinal epithelial cells secrete a wide variety of chemokines, often in response to agents present in the intestinal lumen. Macrophage inflammatory protein 2 (MIP-2) is a chemokine that attracts neutrophils, and its secretion from intestinal epithelial cells is enhanced by inflammatory stimuli such as interleukin 1β. We hypothesised that the production of MIP-2 by epithelial cells would increase leucocyte migration into the intestine. AIM—To study the effects of a chemokine secreted from intestinal epithelial cells in vivo. METHODS—MIP-2 was expressed in the mouse intestinal epithelium using an epithelial cell specific promoter from the gene encoding the intestinal fatty acid binding protein. The intestines of these transgenic mice were then analysed. RESULTS—Epithelial cells from transgenic mice expressed MIP-2 but wild-type mice did not. Neutrophil recruitment, examined by myeloperoxidase (MPO) staining and total MPO activity per unit weight of intestine, was significantly increased in transgenic mice in both the small intestine and proximal colon, and this was blocked by anti-MIP-2 antibody treatment. Both intraepithelial and lamina propria lymphocytes were also increased in transgenic mice. They showed chemotactic activity to MIP-2 in the Boyden chambers and expressed MIP-2 receptor (CXCR-2) mRNA confirmed by reverse transcription-polymerase chain reaction. CONCLUSION—These experiments are the first to show a functional role for epithelial chemokines in vivo and reveal an unexpected role for the neutrophil chemokine MIP-2 in controlling mucosal lymphocyte migration. Keywords: chemokines; epithelial cells; intestinal mucosa; leucocyte recruitment; transgenic mouse
Full Text
The Full Text of this article is available as a PDF (222.6 KB).
Figure 1 .
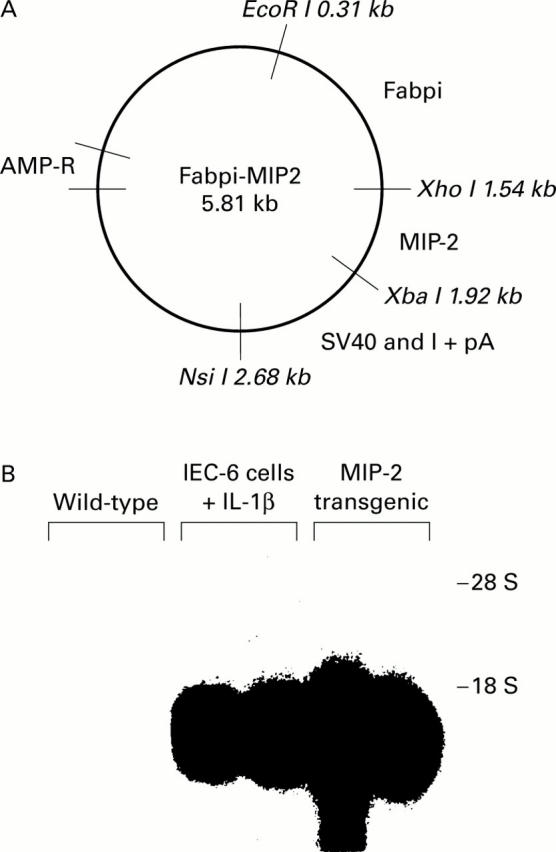
Generation of macrophage inflammatory protein 2 (MIP-2) transgenic mice. (A) Diagrammatic representation of the plasmid containing the fatty acid binding protein of the intestine (Fabpi) promoter linked to the MIP-2 gene and the SV40 intron and polyadenylation site. MIP-2 cDNA was derived from a mouse MIP-2 sequence using polymerase chain reaction with primers synthesised with a Xho I site and an Xba I site. (B) Northern blot analysis of MIP-2 mRNA accumulation in the intestinal epithelium of wild-type and transgenic mice. MIP-2 mRNA was detected using an MIP-2 cDNA probe. Epithelial cells were removed from wild-type mice (lanes 1, 2) and from transgenic mice (lanes 5, 6). MIP-2 mRNA derived from IEC-6 cells stimulated with interleukin 1β (IL-1β) was used as a positive control (lanes 3, 4).
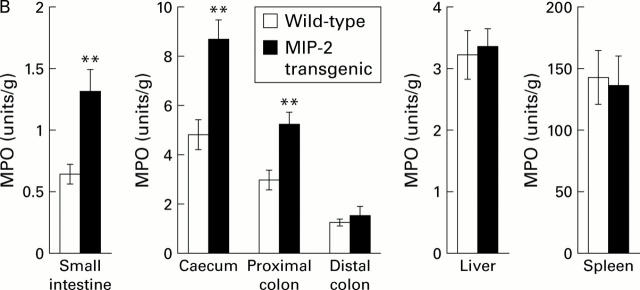
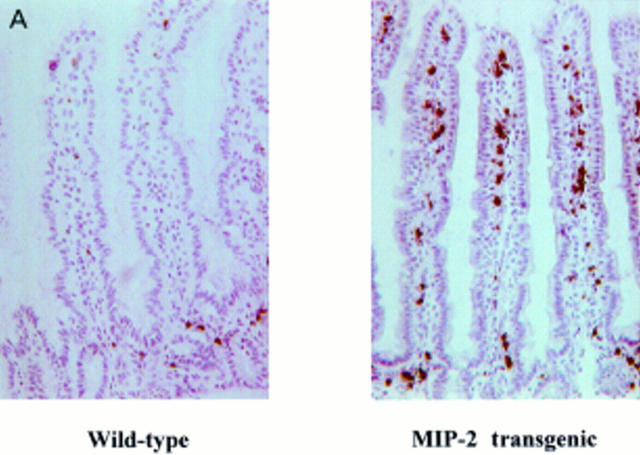
Figure 2 .
Neutrophil recruitment increases in macrophage inflammatory protein 2 (MIP-2) transgenic mice. (A) Neutrophils in the mid jejunum were stained by myeloperoxidase (MPO) using the Hanker Yates' reaction. Magnification ×100. (B) Neutrophil recruitment is demonstrated by total MPO activity. MPO was extracted with 0.5% hexadecyltrimethylammonium bromide. Total MPO activity is expressed per unit weight of tissue derived from wild-type MIP-2 transgenic mice. Expression of MPO activity as a proportion of unit per weight of whole tissue appeared to be smaller in the small intestine than in the colon due to the heavier weight per unit length of the small intestine. Data are presented as mean (SD) of eight animals for each group. **p<0.01 versus wild-type.
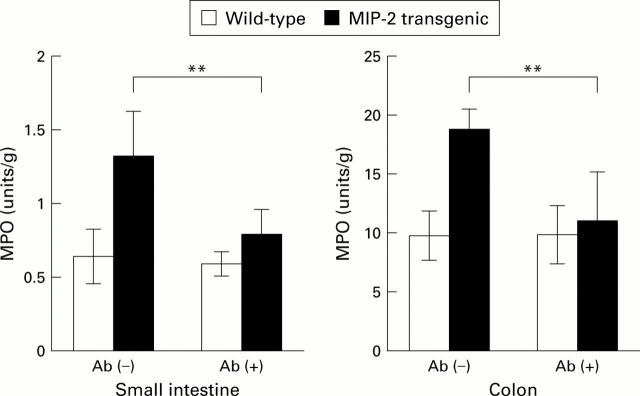
Figure 3 .
Neutrophil recruitment due to macrophage inflammatory protein 2 (MIP-2) secretion was blocked with an anti-MIP-2 antibody. Anti-MIP-2 antibody was injected intravenously seven days and four days before examining the mice. Neutrophil recruitment was examined by measuring myeloperoxidase (MPO) activity per unit weight of tissue samples derived from wild-type and MIP-2 transgenic mice. Ab (−), mice received phosphate buffered saline only; Ab (+), mice received anti-MIP-2 antibody treatment. Data are presented as mean (SD) of eight animals for each group. **p<0.01.
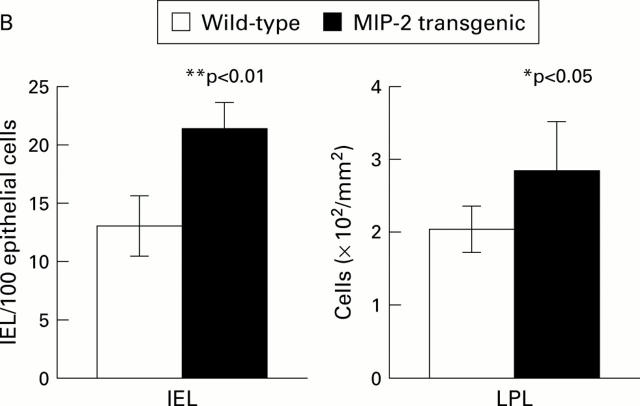
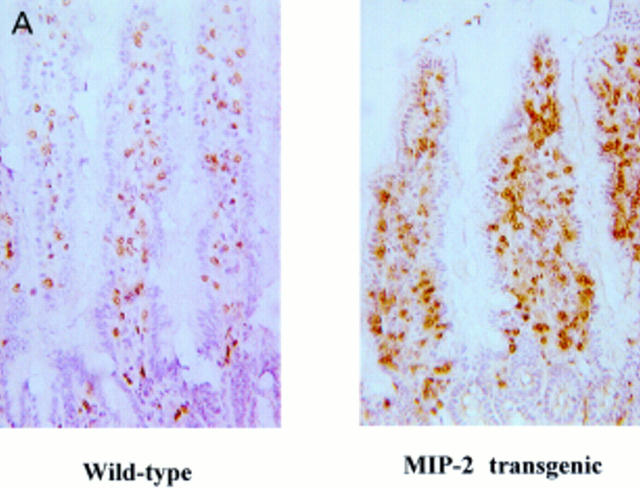
Figure 4 .
Intraepithelial lymphocyte (IEL) and lamina propria lymphocyte (LPL) numbers were increased in the small intestine of macrophage inflammatory protein 2 (MIP-2) transgenic mice. (A) Lymphocytes were detected with an anti-CD3 Ab (145-2C11). Magnification ×100. (B) IELs were expressed as number of CD3 positive cells in the epithelium per 100 epithelial cells. LPLs were expressed as CD3 positive cells in the lamina propria per mm2. Data are presented as mean (SD) of six animals for each group. *p<0.05, **p<0.01.
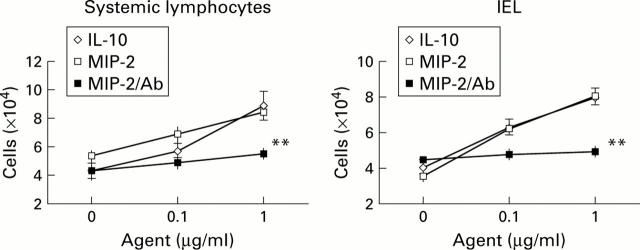
Figure 5 .
Macrophage inflammatory protein 2 (MIP-2) attracts lymphocytes, including intraepithelial lymphocytes (IEL) in vitro. Lymphocyte migration in response to interleukin 10 (IL-10) and MIP-2 in the absence or presence of anti-MIP-2 antibody (MIP-2/Ab) was studied using the Boyden chamber. Lymphocytes (2×105/0.2 ml) were placed in the upper chambers, and migrated cells were counted after four hours of incubation at 37°C. Each experiment was done in duplicate. Data are presented as mean (SEM) of six animals for each group. Significant differences were evaluated in assays between supernatants with or without anti-MIP-2 antibody. **p<0.01.
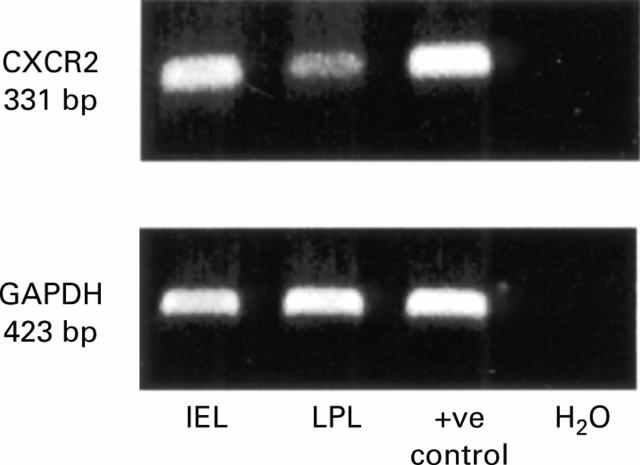
Figure 6 .
Analysis of CXCR-2 expression on lymphocytes from wild-type mice. Agarose gel stained with ethidium bromide showing reverse transcription-polymerase chain reaction (RT-PCR) products for CXCR-2 (331 bp) and GAPDH (423 bp). Expression of CXCR-2 and GAPDH mRNA was confirmed in intraepithelial lymphocytes (IEL) and lamina propria lymphocytes (LPL) isolated from wild-type mice. RNA extracted from whole spleen was used as a positive control and PCR mixture without RNA but distilled H2O was used as a negative control. Representative of three independent experiments.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baetscher M., Schmidt E., Shimizu A., Leder P., Fishman M. C. SV40 T antigen transforms calcitonin cells of the thyroid but not CGRP-containing neurons in transgenic mice. Oncogene. 1991 Jul;6(7):1133–1138. [PubMed] [Google Scholar]
- Cicalese L., Caraceni P., Nalesnik M. A., Borle A. B., Schraut W. H. Oxygen free radical content and neutrophil infiltration are important determinants in mucosal injury after rat small bowel transplantation. Transplantation. 1996 Jul 27;62(2):161–166. doi: 10.1097/00007890-199607270-00003. [DOI] [PubMed] [Google Scholar]
- Cohn S. M., Simon T. C., Roth K. A., Birkenmeier E. H., Gordon J. I. Use of transgenic mice to map cis-acting elements in the intestinal fatty acid binding protein gene (Fabpi) that control its cell lineage-specific and regional patterns of expression along the duodenal-colonic and crypt-villus axes of the gut epithelium. J Cell Biol. 1992 Oct;119(1):27–44. doi: 10.1083/jcb.119.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe S. E., Alvarez L., Dytoc M., Hunt R. H., Muller M., Sherman P., Patel J., Jin Y., Ernst P. B. Expression of interleukin 8 and CD54 by human gastric epithelium after Helicobacter pylori infection in vitro. Gastroenterology. 1995 Jan;108(1):65–74. doi: 10.1016/0016-5085(95)90009-8. [DOI] [PubMed] [Google Scholar]
- Driscoll K. E., Howard B. W., Carter J. M., Asquith T., Johnston C., Detilleux P., Kunkel S. L., Isfort R. J. Alpha-quartz-induced chemokine expression by rat lung epithelial cells: effects of in vivo and in vitro particle exposure. Am J Pathol. 1996 Nov;149(5):1627–1637. [PMC free article] [PubMed] [Google Scholar]
- Eckmann L., Jung H. C., Schürer-Maly C., Panja A., Morzycka-Wroblewska E., Kagnoff M. F. Differential cytokine expression by human intestinal epithelial cell lines: regulated expression of interleukin 8. Gastroenterology. 1993 Dec;105(6):1689–1697. doi: 10.1016/0016-5085(93)91064-o. [DOI] [PubMed] [Google Scholar]
- Fusunyan R. D., Quinn J. J., Ohno Y., MacDermott R. P., Sanderson I. R. Butyrate enhances interleukin (IL)-8 secretion by intestinal epithelial cells in response to IL-1beta and lipopolysaccharide. Pediatr Res. 1998 Jan;43(1):84–90. doi: 10.1203/00006450-199801000-00013. [DOI] [PubMed] [Google Scholar]
- Green R. P., Cohn S. M., Sacchettini J. C., Jackson K. E., Gordon J. I. The mouse intestinal fatty acid binding protein gene: nucleotide sequence, pattern of developmental and regional expression, and proposed structure of its protein product. DNA Cell Biol. 1992 Jan-Feb;11(1):31–41. doi: 10.1089/dna.1992.11.31. [DOI] [PubMed] [Google Scholar]
- Guix M., Skinner J. M., Whitehead R. Measuring intraepithelial lymphocytes, surface area, and volume of lamina propria in the jejunal mucosa of coeliac patients. Gut. 1979 Apr;20(4):275–278. doi: 10.1136/gut.20.4.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann A., Andrew D. P., Jablonski-Westrich D., Holzmann B., Butcher E. C. Role of alpha 4-integrins in lymphocyte homing to mucosal tissues in vivo. J Immunol. 1994 Apr 1;152(7):3282–3293. [PubMed] [Google Scholar]
- Hermiston M. L., Gordon J. I. Inflammatory bowel disease and adenomas in mice expressing a dominant negative N-cadherin. Science. 1995 Nov 17;270(5239):1203–1207. doi: 10.1126/science.270.5239.1203. [DOI] [PubMed] [Google Scholar]
- Hershberg R. M., Cho D. H., Youakim A., Bradley M. B., Lee J. S., Framson P. E., Nepom G. T. Highly polarized HLA class II antigen processing and presentation by human intestinal epithelial cells. J Clin Invest. 1998 Aug 15;102(4):792–803. doi: 10.1172/JCI3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung H. C., Eckmann L., Yang S. K., Panja A., Fierer J., Morzycka-Wroblewska E., Kagnoff M. F. A distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion. J Clin Invest. 1995 Jan;95(1):55–65. doi: 10.1172/JCI117676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick B. A., Parkos C. A., Colgan S. P., Carnes D. K., Madara J. L. Apical secretion of a pathogen-elicited epithelial chemoattractant activity in response to surface colonization of intestinal epithelia by Salmonella typhimurium. J Immunol. 1998 Jan 1;160(1):455–466. [PubMed] [Google Scholar]
- Nielsen O. H., Rüdiger N., Gaustadnes M., Horn T. Intestinal interleukin-8 concentration and gene expression in inflammatory bowel disease. Scand J Gastroenterol. 1997 Oct;32(10):1028–1034. doi: 10.3109/00365529709011220. [DOI] [PubMed] [Google Scholar]
- Ohno Y., Lee J., Fusunyan R. D., MacDermott R. P., Sanderson I. R. Macrophage inflammatory protein-2: chromosomal regulation in rat small intestinal epithelial cells. Proc Natl Acad Sci U S A. 1997 Sep 16;94(19):10279–10284. doi: 10.1073/pnas.94.19.10279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsuka Y., Yamashiro Y., Maeda M., Oguchi S., Shimizu T., Nagata S., Yagita H., Yabuta K., Okumura K. Food antigen activates intraepithelial and lamina propria lymphocytes in food-sensitive enteropathy in mice. Pediatr Res. 1996 May;39(5):862–866. doi: 10.1203/00006450-199605000-00020. [DOI] [PubMed] [Google Scholar]
- Phillips A. D., Rice S. J., France N. E., Walker-Smith J. A. Small intestinal intraepithelial lymphocyte levels in cow's milk protein intolerance. Gut. 1979 Jun;20(6):509–512. doi: 10.1136/gut.20.6.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A. I., Bilenker M., Ebert E. C. Intestinal intraepithelial lymphocytes have a promiscuous interleukin-8 receptor. Gut. 1997 Mar;40(3):333–338. doi: 10.1136/gut.40.3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson I. R. Dietary regulation of genes expressed in the developing intestinal epithelium. Am J Clin Nutr. 1998 Nov;68(5):999–1005. doi: 10.1093/ajcn/68.5.999. [DOI] [PubMed] [Google Scholar]
- Sanderson I. R., Ouellette A. J., Carter E. A., Harmatz P. R. Ontogeny of Ia messenger RNA in the mouse small intestinal epithelium is modulated by age of weaning and diet. Gastroenterology. 1993 Oct;105(4):974–980. doi: 10.1016/0016-5085(93)90939-a. [DOI] [PubMed] [Google Scholar]
- Sanderson I. R., Ouellette A. J., Carter E. A., Walker W. A., Harmatz P. R. Differential regulation of B7 mRNA in enterocytes and lymphoid cells. Immunology. 1993 Jul;79(3):434–438. [PMC free article] [PubMed] [Google Scholar]
- Simon T. C., Roberts L. J., Gordon J. I. A 20-nucleotide element in the intestinal fatty acid binding protein gene modulates its cell lineage-specific, differentiation-dependent, and cephalocaudal patterns of expression in transgenic mice. Proc Natl Acad Sci U S A. 1995 Sep 12;92(19):8685–8689. doi: 10.1073/pnas.92.19.8685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tani K., Su S. B., Utsunomiya I., Oppenheim J. J., Wang J. M. Interferon-gamma maintains the binding and functional capacity of receptors for IL-8 on cultured human T cells. Eur J Immunol. 1998 Feb;28(2):502–507. doi: 10.1002/(SICI)1521-4141(199802)28:02<502::AID-IMMU502>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Tekamp-Olson P., Gallegos C., Bauer D., McClain J., Sherry B., Fabre M., van Deventer S., Cerami A. Cloning and characterization of cDNAs for murine macrophage inflammatory protein 2 and its human homologues. J Exp Med. 1990 Sep 1;172(3):911–919. doi: 10.1084/jem.172.3.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M., Ueno Y., Yajima T., Iwao Y., Tsuchiya M., Ishikawa H., Aiso S., Hibi T., Ishii H. Interleukin 7 is produced by human intestinal epithelial cells and regulates the proliferation of intestinal mucosal lymphocytes. J Clin Invest. 1995 Jun;95(6):2945–2953. doi: 10.1172/JCI118002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein D. L., O'Neill B. L., Metcalf E. S. Salmonella typhi stimulation of human intestinal epithelial cells induces secretion of epithelial cell-derived interleukin-6. Infect Immun. 1997 Feb;65(2):395–404. doi: 10.1128/iai.65.2.395-404.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpe S. D., Davatelis G., Sherry B., Beutler B., Hesse D. G., Nguyen H. T., Moldawer L. L., Nathan C. F., Lowry S. F., Cerami A. Macrophages secrete a novel heparin-binding protein with inflammatory and neutrophil chemokinetic properties. J Exp Med. 1988 Feb 1;167(2):570–581. doi: 10.1084/jem.167.2.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpe S. D., Sherry B., Juers D., Davatelis G., Yurt R. W., Cerami A. Identification and characterization of macrophage inflammatory protein 2. Proc Natl Acad Sci U S A. 1989 Jan;86(2):612–616. doi: 10.1073/pnas.86.2.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki Y., Matsuo Y., Zagorski J., Matsuura N., Onodera H., Itoyama Y., Kogure K. New therapeutic possibility of blocking cytokine-induced neutrophil chemoattractant on transient ischemic brain damage in rats. Brain Res. 1997 Jun 6;759(1):103–111. doi: 10.1016/s0006-8993(97)00251-5. [DOI] [PubMed] [Google Scholar]
- Yang S. K., Eckmann L., Panja A., Kagnoff M. F. Differential and regulated expression of C-X-C, C-C, and C-chemokines by human colon epithelial cells. Gastroenterology. 1997 Oct;113(4):1214–1223. doi: 10.1053/gast.1997.v113.pm9322516. [DOI] [PubMed] [Google Scholar]
- Z'Graggen K., Walz A., Mazzucchelli L., Strieter R. M., Mueller C. The C-X-C chemokine ENA-78 is preferentially expressed in intestinal epithelium in inflammatory bowel disease. Gastroenterology. 1997 Sep;113(3):808–816. doi: 10.1016/s0016-5085(97)70175-6. [DOI] [PubMed] [Google Scholar]