Abstract
BACKGROUND—Crohn's disease (CD) is a chronic relapsing inflammatory bowel disease of unknown origin. It is characterised by chronic mucosal ulcerations which affect any part of the intestine but most commonly are found in the ileum and proximal colon. AIMS—Studies were undertaken to provide information regarding cell specific expression of mucin genes in the ileum of patients with CD. PATIENTS AND METHODS—Expression of mucin genes was analysed in the ileal mucosa of patients with CD and controls by in situ hybridisation and immunohistochemistry. RESULTS—In healthy ileal mucosa, patients with CD showed a pattern identical to normal controls with main expression of MUC2 and MUC3, lesser expression of MUC1 and MUC4, and no expression of MUC5AC, MUC5B, MUC6, or MUC7. In the involved mucosa, the pattern was somewhat comparable although heterogeneous to that observed in healthy ileal mucosa. Importantly, a particular mucin gene expression pattern was observed in ileal mucosa close to the ulcer margins in ulcer associated cell lineage, with the appearance of MUC5AC and MUC6 mRNAs and peptides, which are normally restricted to the stomach (MUC5AC and MUC6) and duodenum (MUC6), and disappearance of MUC2. CONCLUSIONS—Our results suggest that gel forming mucins (more particularly MUC5AC and MUC6) may have a role in epithelial wound healing after mucosal injury in inflammatory bowel diseases in addition to mucosal protection. Keywords: mucins; MUC genes; Crohn's disease; ulcer associated cell lineage
Full Text
The Full Text of this article is available as a PDF (281.6 KB).
Figure 1 .
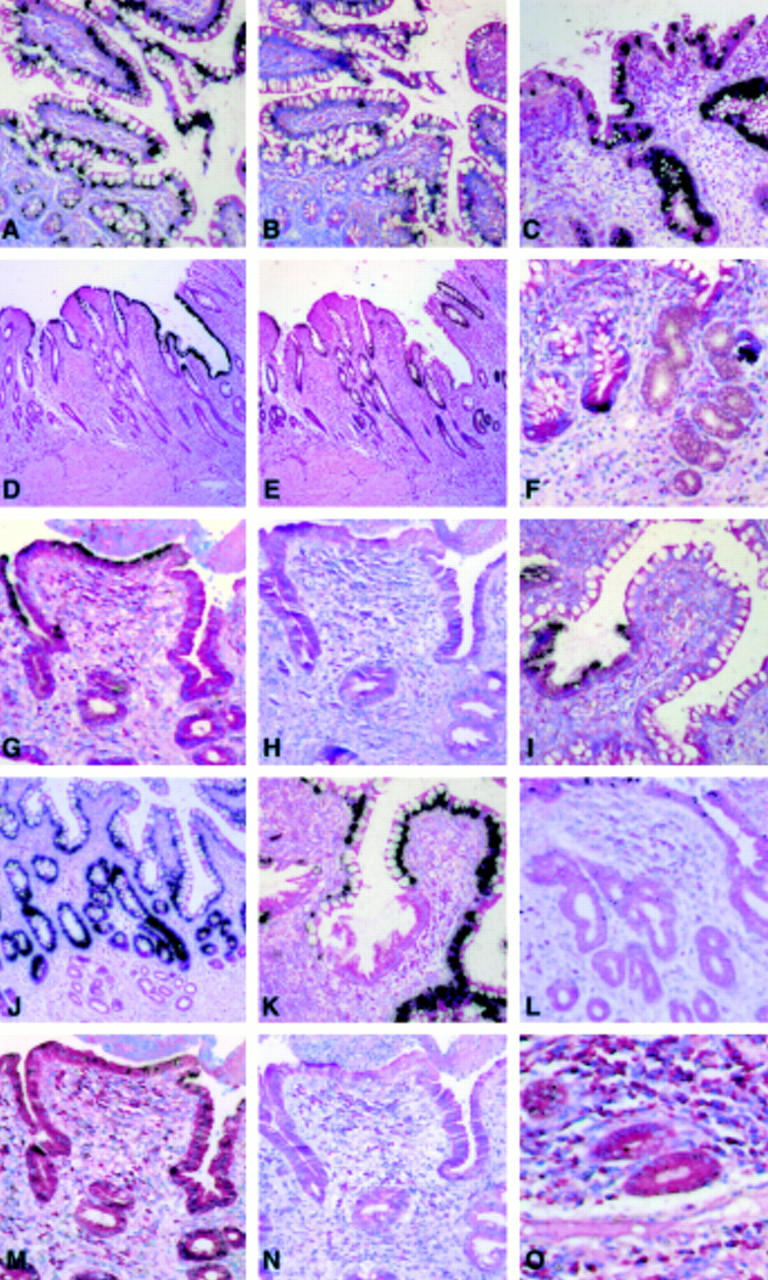
In situ hybridisation for mucin gene mRNAs in ileal mucosa of patients with Crohn's disease. (A, B) In situ hybridisation for MUC2 (A) and MUC3 (B) mRNAs in healthy ileal mucosa. (A) With the MUC2 probe, labelling was strong and located in the perinuclear region of goblet cells both on villi and in crypts whereas in (B) with the MUC3 probe, labelling was of moderate intensity and located in goblet and absorptive cells on villi (magnification ×200). (C-E) In situ hybridisation for MUC2 (C), MUC3 (D), and MUC4 (E) mRNAs in involved ileal mucosa showing the heterogeneity of the labelling in a given specimen (magnification (C) ×200, (D and E) ×100). (F-O) In situ hybridisation for MUC6 (F), MUC5AC (G-I), MUC2 (J, K), MUC4 (L), MUC3 (M, N), and MUC1 (O) mRNAs in involved ileal mucosa adjacent to ulcerations in the ulcer associated cell lineage (UACL). (F) MUC6 mRNA was observed in newly formed acinar glands of the lineage. (G-I) MUC5AC mRNA was observed in epithelial cells of the upper part of the ducts that develop eventually from the new glands and in epithelial cells that have reached the luminal surface and migrate onto the villus surface with the 35S labelled MUC5AC probe (G, I) whereas hybridisation signal was absent with the 35S labelled MUC5AC probe and a large excess of unlabelled MUC5AC probe (H) (negative control). (J, K) MUC2 mRNA was not detected in acinar (J) or surface cells (K) of the new lineage whereas a strong signal was observed in the surrounding goblet cells. (L) A weak signal was observed with the MUC4 probe in epithelial cells of the surface and the ducts. (M, N) A weak signal was observed in epithelial cells of the surface and the ducts with the 35S labelled MUC3 probe (M) whereas hybridisation signal was absent with the 35S labelled MUC3 probe and a large excess of unlabelled MUC3 probe (N) (negative control). (O) A weak signal was observed with the MUC1 probe in epithelial cells of the UACL (magnification (F-I, K-N) ×200, (J) ×100, (O) ×400; all sections were counterstained with methyl green pyronin).
Figure 2 .
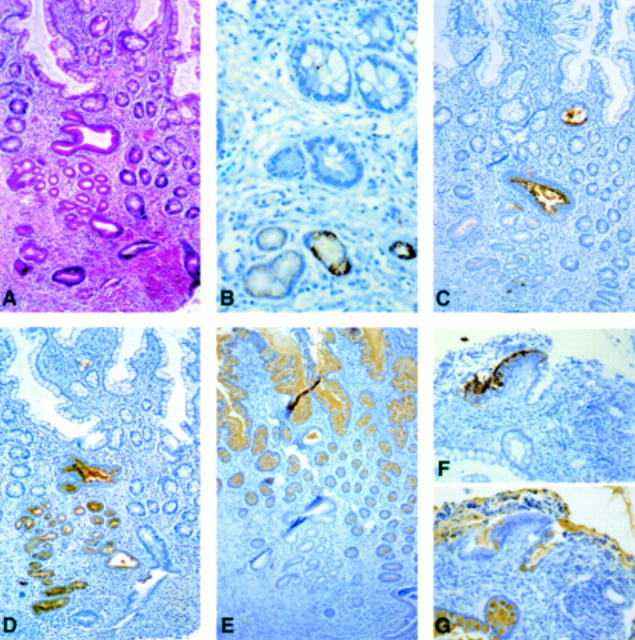
Immunohistochemistry for mucin gene peptides in involved ileal mucosa of patients with Crohn's disease. (A) Involved ileal mucosa adjacent to an ulceration stained with haematoxylin-eosin-saffron and astra blue showing the UACL (magnification ×100). (B-G) Immunohistochemistry for MUC6 (B), MUC5AC (C, F), MUC5B (D), and MUC2 (E, G) in the ulcer associated cell lineage (UACL). (B) MUC6 peptides were observed in acinar cells whereas (C and F) MUC5AC was present in surface cells; MUC5B peptides were observed throughout the UACL; (E, G) MUC2 peptides were not detected in acinar (E) or surface cells (G) of the new lineage whereas strong staining was observed in the surrounding goblet cells (magnification (B) ×400, (C-E) ×100, (F, G) ×250; all sections were counterstained with haematoxylin).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahnen D. J., Poulsom R., Stamp G. W., Elia G., Pike C., Jeffery R., Longcroft J., Rio M. C., Chambon P., Wright N. A. The ulceration-associated cell lineage (UACL) reiterates the Brunner's gland differentiation programme but acquires the proliferative organization of the gastric gland. J Pathol. 1994 Aug;173(4):317–326. doi: 10.1002/path.1711730406. [DOI] [PubMed] [Google Scholar]
- Audie J. P., Janin A., Porchet N., Copin M. C., Gosselin B., Aubert J. P. Expression of human mucin genes in respiratory, digestive, and reproductive tracts ascertained by in situ hybridization. J Histochem Cytochem. 1993 Oct;41(10):1479–1485. doi: 10.1177/41.10.8245407. [DOI] [PubMed] [Google Scholar]
- Bartman A. E., Buisine M. P., Aubert J. P., Niehans G. A., Toribara N. W., Kim Y. S., Kelly E. J., Crabtree J. E., Ho S. B. The MUC6 secretory mucin gene is expressed in a wide variety of epithelial tissues. J Pathol. 1998 Dec;186(4):398–405. doi: 10.1002/(SICI)1096-9896(199812)186:4<398::AID-PATH192>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Buisine M. P., Desreumaux P., Debailleul V., Gambiez L., Geboes K., Ectors N., Delescaut M. P., Degand P., Aubert J. P., Colombel J. F. Abnormalities in mucin gene expression in Crohn's disease. Inflamm Bowel Dis. 1999 Feb;5(1):24–32. doi: 10.1097/00054725-199902000-00004. [DOI] [PubMed] [Google Scholar]
- Buisine M. P., Devisme L., Copin M. C., Durand-Réville M., Gosselin B., Aubert J. P., Porchet N. Developmental mucin gene expression in the human respiratory tract. Am J Respir Cell Mol Biol. 1999 Feb;20(2):209–218. doi: 10.1165/ajrcmb.20.2.3259. [DOI] [PubMed] [Google Scholar]
- Buisine M. P., Devisme L., Degand P., Dieu M. C., Gosselin B., Copin M. C., Aubert J. P., Porchet N. Developmental mucin gene expression in the gastroduodenal tract and accessory digestive glands. II. Duodenum and liver, gallbladder, and pancreas. J Histochem Cytochem. 2000 Dec;48(12):1667–1676. doi: 10.1177/002215540004801210. [DOI] [PubMed] [Google Scholar]
- Buisine M. P., Devisme L., Maunoury V., Deschodt E., Gosselin B., Copin M. C., Aubert J. P., Porchet N. Developmental mucin gene expression in the gastroduodenal tract and accessory digestive glands. I. Stomach. A relationship to gastric carcinoma. J Histochem Cytochem. 2000 Dec;48(12):1657–1666. doi: 10.1177/002215540004801209. [DOI] [PubMed] [Google Scholar]
- Buisine M. P., Devisme L., Savidge T. C., Gespach C., Gosselin B., Porchet N., Aubert J. P. Mucin gene expression in human embryonic and fetal intestine. Gut. 1998 Oct;43(4):519–524. doi: 10.1136/gut.43.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corfield A. P., Warren B. F. Mucus glycoproteins and their role in colorectal disease. J Pathol. 1996 Sep;180(1):8–17. doi: 10.1002/(SICI)1096-9896(199609)180:1<8::AID-PATH596>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- D'Haens G., Geboes K., Ponette E., Penninckx F., Rutgeerts P. Healing of severe recurrent ileitis with azathioprine therapy in patients with Crohn's disease. Gastroenterology. 1997 May;112(5):1475–1481. doi: 10.1016/s0016-5085(97)70027-1. [DOI] [PubMed] [Google Scholar]
- Dignass A., Lynch-Devaney K., Kindon H., Thim L., Podolsky D. K. Trefoil peptides promote epithelial migration through a transforming growth factor beta-independent pathway. J Clin Invest. 1994 Jul;94(1):376–383. doi: 10.1172/JCI117332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geboes K., Desreumaux P., Jouret A., Ectors N., Rutgeerts P., Colombel J. F. Diagnostic histopathologique de l'activité des maladies inflammatoires chroniques de l'intestin. Evaluation de l'effet des traitements médicamenteux. Application des scores histologiques. Gastroenterol Clin Biol. 1999 Oct;23(10):1062–1073. [PubMed] [Google Scholar]
- Gendler S. J., Spicer A. P. Epithelial mucin genes. Annu Rev Physiol. 1995;57:607–634. doi: 10.1146/annurev.ph.57.030195.003135. [DOI] [PubMed] [Google Scholar]
- Gower-Rousseau C., Salomez J. L., Dupas J. L., Marti R., Nuttens M. C., Votte A., Lemahieu M., Lemaire B., Colombel J. F., Cortot A. Incidence of inflammatory bowel disease in northern France (1988-1990). Gut. 1994 Oct;35(10):1433–1438. doi: 10.1136/gut.35.10.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanby A. M., Poulsom R., Singh S., Elia G., Jeffery R. E., Wright N. A. Spasmolytic polypeptide is a major antral peptide: distribution of the trefoil peptides human spasmolytic polypeptide and pS2 in the stomach. Gastroenterology. 1993 Oct;105(4):1110–1116. doi: 10.1016/0016-5085(93)90956-d. [DOI] [PubMed] [Google Scholar]
- Hauser F., Poulsom R., Chinery R., Rogers L. A., Hanby A. M., Wright N. A., Hoffmann W. hP1.B, a human P-domain peptide homologous with rat intestinal trefoil factor, is expressed also in the ulcer-associated cell lineage and the uterus. Proc Natl Acad Sci U S A. 1993 Aug 1;90(15):6961–6965. doi: 10.1073/pnas.90.15.6961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho S. B., Shekels L. L., Toribara N. W., Kim Y. S., Lyftogt C., Cherwitz D. L., Niehans G. A. Mucin gene expression in normal, preneoplastic, and neoplastic human gastric epithelium. Cancer Res. 1995 Jun 15;55(12):2681–2690. [PubMed] [Google Scholar]
- Hovenberg H. W., Davies J. R., Herrmann A., Lindén C. J., Carlstedt I. MUC5AC, but not MUC2, is a prominent mucin in respiratory secretions. Glycoconj J. 1996 Oct;13(5):839–847. doi: 10.1007/BF00702348. [DOI] [PubMed] [Google Scholar]
- Kindon H., Pothoulakis C., Thim L., Lynch-Devaney K., Podolsky D. K. Trefoil peptide protection of intestinal epithelial barrier function: cooperative interaction with mucin glycoprotein. Gastroenterology. 1995 Aug;109(2):516–523. doi: 10.1016/0016-5085(95)90340-2. [DOI] [PubMed] [Google Scholar]
- Klein O., Colombel J. F., Lescut D., Gambiez L., Desreumaux P., Quandalle P., Cortot A. Remaining small bowel endoscopic lesions at surgery have no influence on early anastomotic recurrences in Crohn's disease. Am J Gastroenterol. 1995 Nov;90(11):1949–1952. [PubMed] [Google Scholar]
- Lapensée L., Paquette Y., Bleau G. Allelic polymorphism and chromosomal localization of the human oviductin gene (MUC9). Fertil Steril. 1997 Oct;68(4):702–708. doi: 10.1016/s0015-0282(97)00317-8. [DOI] [PubMed] [Google Scholar]
- Lesuffleur T., Zweibaum A., Real F. X. Mucins in normal and neoplastic human gastrointestinal tissues. Crit Rev Oncol Hematol. 1994 Dec;17(3):153–180. doi: 10.1016/1040-8428(94)90053-1. [DOI] [PubMed] [Google Scholar]
- Longman R. J., Douthwaite J., Sylvester P. A., Poulsom R., Corfield A. P., Thomas M. G., Wright N. A. Coordinated localisation of mucins and trefoil peptides in the ulcer associated cell lineage and the gastrointestinal mucosa. Gut. 2000 Dec;47(6):792–800. doi: 10.1136/gut.47.6.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Vilar J., Hill R. L. The structure and assembly of secreted mucins. J Biol Chem. 1999 Nov 5;274(45):31751–31754. doi: 10.1074/jbc.274.45.31751. [DOI] [PubMed] [Google Scholar]
- Pigny P., Guyonnet-Duperat V., Hill A. S., Pratt W. S., Galiegue-Zouitina S., d'Hooge M. C., Laine A., Van-Seuningen I., Degand P., Gum J. R. Human mucin genes assigned to 11p15.5: identification and organization of a cluster of genes. Genomics. 1996 Dec 15;38(3):340–352. doi: 10.1006/geno.1996.0637. [DOI] [PubMed] [Google Scholar]
- Podolsky D. K., Lynch-Devaney K., Stow J. L., Oates P., Murgue B., DeBeaumont M., Sands B. E., Mahida Y. R. Identification of human intestinal trefoil factor. Goblet cell-specific expression of a peptide targeted for apical secretion. J Biol Chem. 1993 Mar 25;268(9):6694–6702. [PubMed] [Google Scholar]
- Reis C. A., David L., Carvalho F., Mandel U., de Bolós C., Mirgorodskaya E., Clausen H., Sobrinho-Simões M. Immunohistochemical study of the expression of MUC6 mucin and co-expression of other secreted mucins (MUC5AC and MUC2) in human gastric carcinomas. J Histochem Cytochem. 2000 Mar;48(3):377–388. doi: 10.1177/002215540004800307. [DOI] [PubMed] [Google Scholar]
- Riddell R. H., Goldman H., Ransohoff D. F., Appelman H. D., Fenoglio C. M., Haggitt R. C., Ahren C., Correa P., Hamilton S. R., Morson B. C. Dysplasia in inflammatory bowel disease: standardized classification with provisional clinical applications. Hum Pathol. 1983 Nov;14(11):931–968. doi: 10.1016/s0046-8177(83)80175-0. [DOI] [PubMed] [Google Scholar]
- Rio M. C., Chenard M. P., Wolf C., Marcellin L., Tomasetto C., Lathe R., Bellocq J. P., Chambon P. Induction of pS2 and hSP genes as markers of mucosal ulceration of the digestive tract. Gastroenterology. 1991 Feb;100(2):375–379. doi: 10.1016/0016-5085(91)90205-y. [DOI] [PubMed] [Google Scholar]
- Roberts I. S., Stoddart R. W. Ulcer-associated cell lineage ('pyloric metaplasia') in Crohn's disease: a lectin histochemical study. J Pathol. 1993 Sep;171(1):13–19. doi: 10.1002/path.1711710105. [DOI] [PubMed] [Google Scholar]
- Sands B. E., Podolsky D. K. The trefoil peptide family. Annu Rev Physiol. 1996;58:253–273. doi: 10.1146/annurev.ph.58.030196.001345. [DOI] [PubMed] [Google Scholar]
- Sartor R. B. Current concepts of the etiology and pathogenesis of ulcerative colitis and Crohn's disease. Gastroenterol Clin North Am. 1995 Sep;24(3):475–507. [PubMed] [Google Scholar]
- Seib T., Blin N., Hilgert K., Seifert M., Theisinger B., Engel M., Dooley S., Zang K. D., Welter C. The three human trefoil genes TFF1, TFF2, and TFF3 are located within a region of 55 kb on chromosome 21q22.3. Genomics. 1997 Feb 15;40(1):200–202. doi: 10.1006/geno.1996.4511. [DOI] [PubMed] [Google Scholar]
- Shankar V., Gilmore M. S., Elkins R. C., Sachdev G. P. A novel human airway mucin cDNA encodes a protein with unique tandem-repeat organization. Biochem J. 1994 Jun 1;300(Pt 2):295–298. doi: 10.1042/bj3000295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S., Podolsky D. K., Engel E., Guth P. H., Kaunitz J. D. Human spasmolytic polypeptide decreases proton permeation through gastric mucus in vivo and in vitro. Am J Physiol. 1997 Jun;272(6 Pt 1):G1473–G1480. doi: 10.1152/ajpgi.1997.272.6.G1473. [DOI] [PubMed] [Google Scholar]
- Thim L. Trefoil peptides: from structure to function. Cell Mol Life Sci. 1997 Dec;53(11-12):888–903. doi: 10.1007/s000180050108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasetto C., Masson R., Linares J. L., Wendling C., Lefebvre O., Chenard M. P., Rio M. C. pS2/TFF1 interacts directly with the VWFC cysteine-rich domains of mucins. Gastroenterology. 2000 Jan;118(1):70–80. doi: 10.1016/s0016-5085(00)70415-x. [DOI] [PubMed] [Google Scholar]
- Tomasetto C., Rio M. C., Gautier C., Wolf C., Hareuveni M., Chambon P., Lathe R. hSP, the domain-duplicated homolog of pS2 protein, is co-expressed with pS2 in stomach but not in breast carcinoma. EMBO J. 1990 Feb;9(2):407–414. doi: 10.1002/j.1460-2075.1990.tb08125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toribara N. W., Ho S. B., Gum E., Gum J. R., Jr, Lau P., Kim Y. S. The carboxyl-terminal sequence of the human secretory mucin, MUC6. Analysis Of the primary amino acid sequence. J Biol Chem. 1997 Jun 27;272(26):16398–16403. doi: 10.1074/jbc.272.26.16398. [DOI] [PubMed] [Google Scholar]
- Van Klinken B. J., Dekker J., Büller H. A., Einerhand A. W. Mucin gene structure and expression: protection vs. adhesion. Am J Physiol. 1995 Nov;269(5 Pt 1):G613–G627. doi: 10.1152/ajpgi.1995.269.5.G613. [DOI] [PubMed] [Google Scholar]
- Weiss A. A., Babyatsky M. W., Ogata S., Chen A., Itzkowitz S. H. Expression of MUC2 and MUC3 mRNA in human normal, malignant, and inflammatory intestinal tissues. J Histochem Cytochem. 1996 Oct;44(10):1161–1166. doi: 10.1177/44.10.8813081. [DOI] [PubMed] [Google Scholar]
- Wickström C., Davies J. R., Eriksen G. V., Veerman E. C., Carlstedt I. MUC5B is a major gel-forming, oligomeric mucin from human salivary gland, respiratory tract and endocervix: identification of glycoforms and C-terminal cleavage. Biochem J. 1998 Sep 15;334(Pt 3):685–693. doi: 10.1042/bj3340685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams S. J., McGuckin M. A., Gotley D. C., Eyre H. J., Sutherland G. R., Antalis T. M. Two novel mucin genes down-regulated in colorectal cancer identified by differential display. Cancer Res. 1999 Aug 15;59(16):4083–4089. [PubMed] [Google Scholar]
- Wright N. A., Pike C., Elia G. Induction of a novel epidermal growth factor-secreting cell lineage by mucosal ulceration in human gastrointestinal stem cells. Nature. 1990 Jan 4;343(6253):82–85. doi: 10.1038/343082a0. [DOI] [PubMed] [Google Scholar]
- Wright N. A., Poulsom R., Stamp G., Van Noorden S., Sarraf C., Elia G., Ahnen D., Jeffery R., Longcroft J., Pike C. Trefoil peptide gene expression in gastrointestinal epithelial cells in inflammatory bowel disease. Gastroenterology. 1993 Jan;104(1):12–20. doi: 10.1016/0016-5085(93)90830-6. [DOI] [PubMed] [Google Scholar]


