Abstract
BACKGROUND AND AIMS—Helicobacter pylori locate not only on the apical surface of surface mucous cells but also in the mucous gel layer covering the gastric mucosa. The present study was undertaken to observe the mucous gel layer itself and any H pylori in this layer at the electron microscopic level, and to determine whether H pylori proliferate in this layer. METHODS—We examined resected human stomachs (five cases, fixed in Carnoy's solution, paraffin embedded) under the light microscope, and gastric biopsy specimens (10 cases, fixed in glutaraldehyde with or without osmium, epoxy embedded) under the electron microscope. We performed histochemical staining for gastric mucins and immunostaining for H pylori, gastric gland mucous type mucins, and intestinal mucins. RESULTS—Under the electron microscope, surface mucous cell type mucins and gland mucous cell type mucins in the mucous gel layer covering gastric mucosa without intestinal metaplasia showed reticular and band like structures, respectively. H pylori were frequently found as small aggregates within the mucous gel layer of surface mucous cell type mucins, and H pylori within these aggregates were seen dividing. H pylori were frequently found in the mucous gel layer of the surface mucous cell type mucins along the border with the layer of gland mucous cell mucins. Occasionally, H pylori were trapped by frayed thin threads of the gland mucous cell type mucins. CONCLUSIONS—The two types of gastric mucins in the mucous gel layer differ in ultrastructure. H pylori preferentially colonise and form microcolonies within the mucous gel layer of surface mucous cell type mucins. Mucins from gland mucous cells may disturb the movement of H pylori within the mucous gel layer. Keywords: gastric mucin; Helicobacter pylori; immunohistochemistry; surface mucous gel layer
Full Text
The Full Text of this article is available as a PDF (328.7 KB).
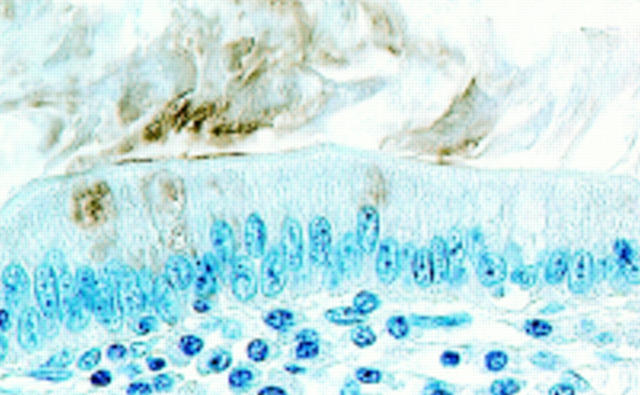
Figure 1 .
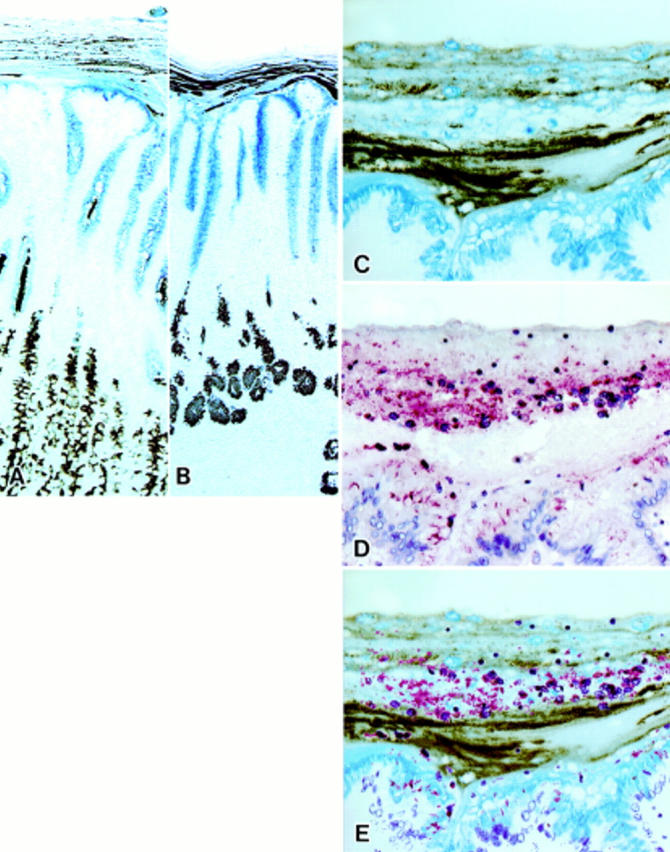
Mucin histochemical appearance of the surface mucous gel layer (SMGL) and immunostaining for Helicobacter pylori on gastric mucosa fixed in Carnoy's solution. (C) and (D) were prepared from serial sections of Carnoy fixed paraffin embedded material. (E) is a composite of (C) and (D) prepared using computer software. (A) Fundic mucosa. The galactose oxidase/thionine Schiff reaction/paradoxical concanavalin A staining (GOTS-PCS) procedure stained surface mucous cells blue and mucous neck cells brown. The SMGL showed a multilaminated structure formed by two types of layers piling up on each other. The layers of gland mucous cell mucins were thinner on the fundic mucosa than on the pyloric mucosa (see (B)). Strands of gland mucous cell mucins were located in the axial region of the foveola (GOTS-PCS staining, original magnification ×50). (B) Pyloric mucosa. GOTS-PCS procedure stained surface mucous cells blue and pyloric gland cells brown. The SMGL showed a similar multilaminated structure to that of the pyloric mucosa (GOTS-PCS staining, original magnification ×50). (C) Higher magnification of the SMGL of the pyloric mucosa. The SMGL showed a multilaminated structure consisting of two types of mucins: (i) surface mucous cell type mucins, which stained blue with GOTS, and (ii) gland mucous cell type mucins, which stained brown with PCS (GOTS-PCS staining, original magnification ×200). (D) H pylori were stained red and were demonstrated not only on the apical and/or lateral surface of the surface mucous cells but also within the SMGL in which H pylori were seen as small aggregates with detached epithelial cells in their centre. Aggregations of H pylori were distributed in layers (immunoalkaline phosphatase staining for H pylori, original magnification ×200). (E) Aggregations of H pylori were mostly distributed in the layers containing mucins derived from surface mucous cells.
Figure 2 .
Pyloric mucosa fixed in Carnoy's solution. Sialosyl-Tn was demonstrated in metaplastic goblet cells and in the surface mucous gel layer covering the area of intestinal metaplasia (immunoperoxidase staining for sialosyl-Tn, original magnification ×200).
Figure 3 .
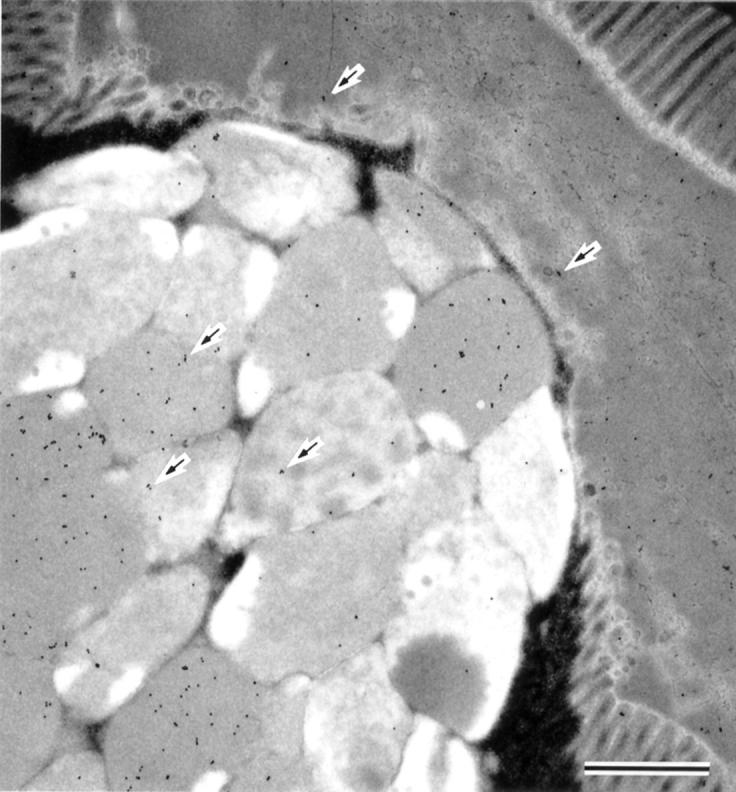
Immunogold staining with antisialosyl-Tn antibody of the area of the intestinal metaplasia. Sialosyl-Tn was demonstrated in the metaplastic goblet cells and in the surface mucous gel layer covering the area of the intestinal metaplasia (arrows) (Immunogold staining with antisialosyl-Tn antibody; bar=1 µm).
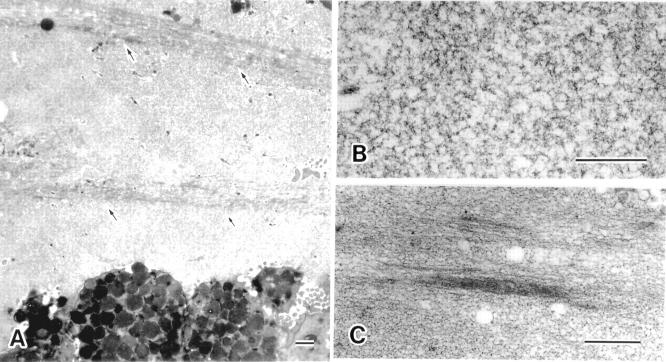
Figure 4 .
Electron microscopic appearance of the surface mucous gel layer (SMGL). (A) Two structural patterns were observed in the SMGL: a reticular pattern (see (B)) and a thick band like pattern (arrows) (see (C)). In this particular view, thick bands running parallel to each other can be seen passing among the mucin threads marking up the reticular pattern. The thickness of the bands varied considerably (bar=1 µm). (B) At higher magnification, mucins with a reticular pattern can be seen to consist of thin threads crossing each other; their diameter was 1.67 (0.69) nm (bar=0.5 µm). (C) At higher magnification, the thick band like mucins appear as an aggregation of thin filamentous threads, approximately 1.07 (0.32) nm in diameter (bar=1 µm).
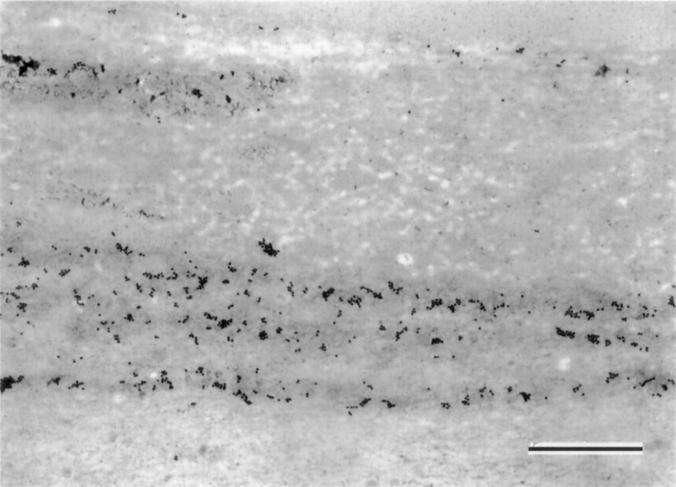
Figure 5 .
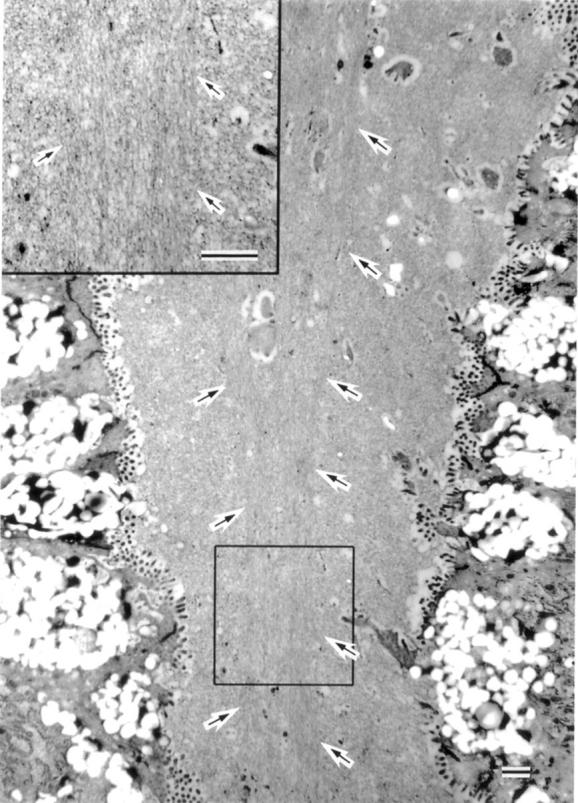
Electron microscopic appearance of mucins in the foveolar lumen. The axial region of the foveolar lumen was occasionally occupied by mucins showing thick threads running parallel with each other (arrows) (bar=1 µm).
Figure 6 .
Surface mucous gel layer Immunogold staining with HIK1083. Mucins of the thick band like pattern were selectively labelled with HIK1083 (Immunogold staining with HIK1083; bar=1 µm).
Figure 7 .
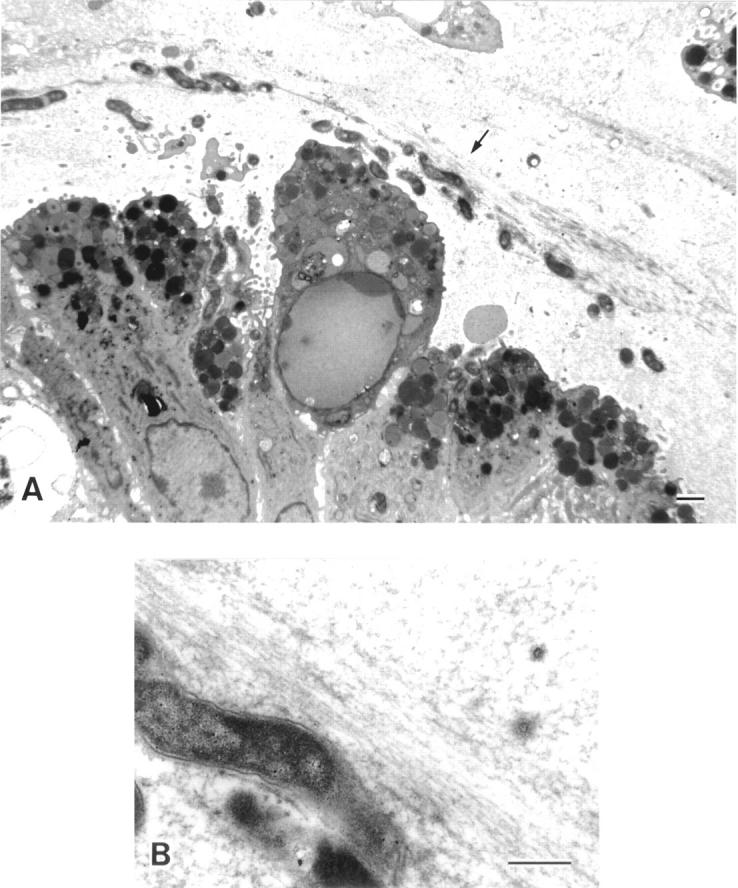
Electron microscopic appearance of Helicobacter pylori infected gastric surface mucous cells. (A) H pylori were also demonstrated on the apical plasma membrane and/or between the lateral plasma membranes of the surface mucous cells. Detaching cells also had H pylori attached to their surface. H pylori located in the reticular pattern layer (arrows) frequently ran parallel to the mucosal surface or to the mucins of the thick band like pattern (bar=1 µm). (B) Higher magnification of H pylori identified with arrows in the surface mucous gel layer in (A) (bar=0.5 µm).
Figure 8 .
Electron microscopic appearance of Helicobacter pylori within the surface mucous gel layer. H pylori were trapped by frayed thin threads of mucins of the thick band like pattern (bar=0.5 µm).
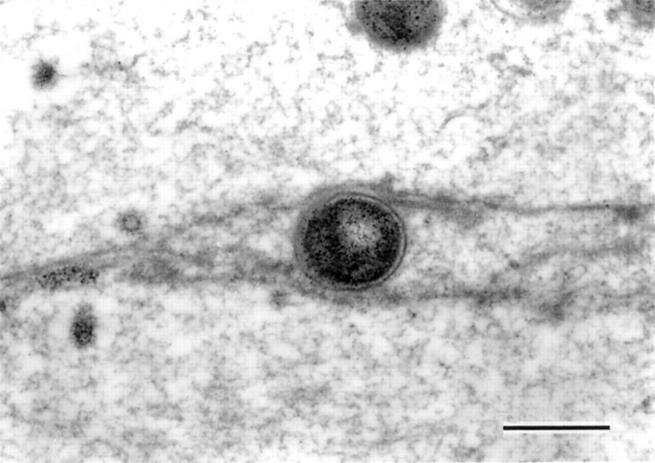
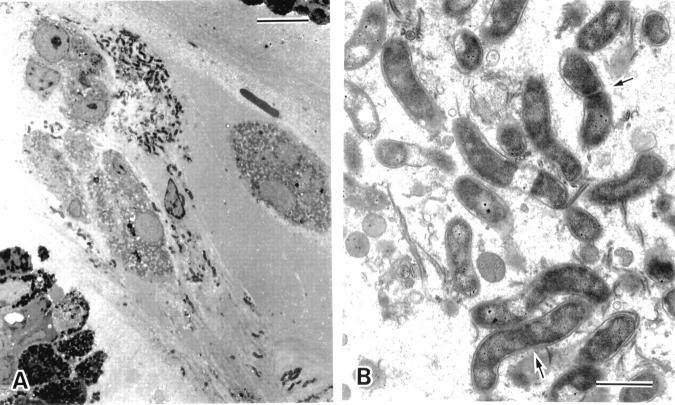
Figure 9 .
Electron microscopic appearance of the aggregates of Helicobacter pylori within the surface mucous gel layer (SMGL). (A) The aggregates of H pylori located in the reticular pattern layer were accompanied by degenerated epithelial cells (bar=10 µm). (B) At higher magnification H pylori showed the typical spiral form with well developed flagellae. Division of the organisms was seen (arrows). Around the microcolonies, the electron density of the SMGL was considerably decreased and the reticular pattern became obscured (see fig 4) (bar=1 µm).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- David L., Nesland J. M., Clausen H., Carneiro F., Sobrinho-Simões M. Simple mucin-type carbohydrate antigens (Tn, sialosyl-Tn and T) in gastric mucosa, carcinomas and metastases. APMIS Suppl. 1992;27:162–172. [PubMed] [Google Scholar]
- Hidaka E., Ota H., Katsuyama T., Nakayama J., Momose M., Hidaka H., Ishii K., Murata F., Tsuyama S., Kurihara M. Coexistence of gland mucous cell-type mucin and lysozyme in gastric gland mucous cells. Histochem Cell Biol. 2000 Feb;113(2):91–98. doi: 10.1007/s004180050011. [DOI] [PubMed] [Google Scholar]
- Ho S. B., Roberton A. M., Shekels L. L., Lyftogt C. T., Niehans G. A., Toribara N. W. Expression cloning of gastric mucin complementary DNA and localization of mucin gene expression. Gastroenterology. 1995 Sep;109(3):735–747. doi: 10.1016/0016-5085(95)90380-1. [DOI] [PubMed] [Google Scholar]
- Ishihara K., Kurihara M., Goso Y., Urata T., Ota H., Katsuyama T., Hotta K. Peripheral alpha-linked N-acetylglucosamine on the carbohydrate moiety of mucin derived from mammalian gastric gland mucous cells: epitope recognized by a newly characterized monoclonal antibody. Biochem J. 1996 Sep 1;318(Pt 2):409–416. doi: 10.1042/bj3180409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuyama T., Spicer S. S. Histochemical differentiation of complex carbohydrates with variants of the concanavalin A-horseradish peroxidase method. J Histochem Cytochem. 1978 Apr;26(4):233–250. doi: 10.1177/26.4.351046. [DOI] [PubMed] [Google Scholar]
- Nakamura N., Ota H., Katsuyama T., Akamatsu T., Ishihara K., Kurihara M., Hotta K. Histochemical reactivity of normal, metaplastic, and neoplastic tissues to alpha-linked N-acetylglucosamine residue-specific monoclonal antibody HIK1083. J Histochem Cytochem. 1998 Jul;46(7):793–801. doi: 10.1177/002215549804600702. [DOI] [PubMed] [Google Scholar]
- Nakayama J., Yeh J. C., Misra A. K., Ito S., Katsuyama T., Fukuda M. Expression cloning of a human alpha1, 4-N-acetylglucosaminyltransferase that forms GlcNAcalpha1-->4Galbeta-->R, a glycan specifically expressed in the gastric gland mucous cell-type mucin. Proc Natl Acad Sci U S A. 1999 Aug 3;96(16):8991–8996. doi: 10.1073/pnas.96.16.8991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota H., Hayama M., Nakayama J., Hidaka H., Honda T., Ishii K., Fukushima M., Uehara T., Kurihara M., Ishihara K. Cell lineage specificity of newly raised monoclonal antibodies against gastric mucins in normal, metaplastic, and neoplastic human tissues and their application to pathology diagnosis. Am J Clin Pathol. 2001 Jan;115(1):69–79. doi: 10.1309/AMUR-K5L3-M2DN-2DK5. [DOI] [PubMed] [Google Scholar]
- Ota H., Katsuyama T. Alternating laminated array of two types of mucin in the human gastric surface mucous layer. Histochem J. 1992 Feb;24(2):86–92. doi: 10.1007/BF01082444. [DOI] [PubMed] [Google Scholar]
- Ota H., Katsuyama T., Ishii K., Nakayama J., Shiozawa T., Tsukahara Y. A dual staining method for identifying mucins of different gastric epithelial mucous cells. Histochem J. 1991 Jan;23(1):22–28. doi: 10.1007/BF01886504. [DOI] [PubMed] [Google Scholar]
- Ota H., Nakayama J., Momose M., Kurihara M., Ishihara K., Hotta K., Katsuyama T. New monoclonal antibodies against gastric gland mucous cell-type mucins: a comparative immunohistochemical study. Histochem Cell Biol. 1998 Aug;110(2):113–119. doi: 10.1007/s004180050272. [DOI] [PubMed] [Google Scholar]
- Shimizu T., Akamatsu T., Sugiyama A., Ota H., Katsuyama T. Helicobacter pylori and the surface mucous gel layer of the human stomach. Helicobacter. 1996 Dec;1(4):207–218. doi: 10.1111/j.1523-5378.1996.tb00041.x. [DOI] [PubMed] [Google Scholar]
- Slomiany B. L., Slomiany A. Mechanism of Helicobacter pylori pathogenesis: focus on mucus. J Clin Gastroenterol. 1992;14 (Suppl 1):S114–S121. [PubMed] [Google Scholar]
- Smith A. W., Chahal B., French G. L. The human gastric pathogen Helicobacter pylori has a gene encoding an enzyme first classified as a mucinase in Vibrio cholerae. Mol Microbiol. 1994 Jul;13(1):153–160. doi: 10.1111/j.1365-2958.1994.tb00410.x. [DOI] [PubMed] [Google Scholar]