Abstract
BACKGROUND—Primary biliary cirrhosis (PBC) is an autoimmune disease in which the pathogenesis of progressive liver injury is poorly understood. AIM—To provide novel insights into the pathogenesis of PBC related liver injury using cDNA array analysis, which simultaneously examines expression of many genes. METHODS—Utilising cDNA arrays of 874 genes, PBC was compared with primary sclerosing cholangitis (PSC) associated cirrhosis and non-diseased liver. Differential expression of 10 genes was confirmed by real time quantitative reverse transcriptase-polymerase chain reaction (RT-PCR). RESULTS—Array analysis identified many differentially expressed genes that are important in inflammation, fibrosis, proliferation, signalling, apoptosis, and oxidative stress. PBC was associated with increased expression of both Th1 and Th2 type molecules of the immune response. Fibrosis related gene expression featured upregulation of connective tissue growth factor and transforming growth factor beta3. Many more apoptosis associated molecules exhibited increased expression, consistent with apoptosis being a more active and regulated process, in PSC associated cirrhosis than in PBC. Increased expression of many genes of the Wnt and notch pathways implicated these highly conserved and linked pathways in PBC pathogenesis. The observed increases in expression of c-jun, c-myc, and c-fos related antigen 1 are consistent with increased Wnt pathway activity in PBC. Differential expression of four components of the Wnt pathway, Wnt-5a, Wnt-13, FRITZ, and beta-catenin, was confirmed by quantitative RT-PCR. CONCLUSION—Many genes implicated in intrahepatic inflammation, fibrosis, and regeneration were upregulated in PBC cirrhosis. In particular, increased expression of a number of Drosophila homologues was seen in PBC. Keywords: primary sclerosing cholangitis; apoptosis; fibrosis; connective tissue growth factor; Wnt; Th1/Th2; brain derived neurotrophic factor; notch
Full Text
The Full Text of this article is available as a PDF (415.7 KB).
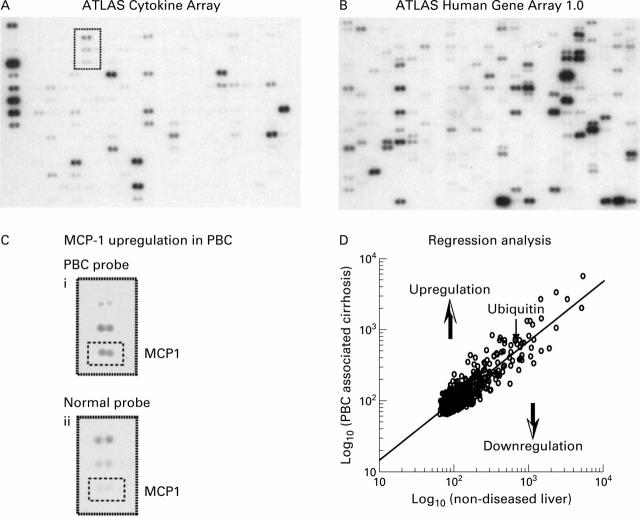
Figure 1 .
Examples of cDNA arrays. ATLAS Cytokine Receptor Array (268 genes and nine housekeeping genes) (A) and ATLAS Human Gene Array 1.0 (588 genes and nine housekeeping genes) (B), both probed with 32P-2' deoxy-cytidine 5' triphosphate (dCTP) labelled primary biliary cirrhosis (PBC) mRNA (pooled from four subjects). The magnified portion of the ATLAS Cytokine Receptor Array compares the 32P-dCTP signal from the PBC and normal liver probes. Upregulation of monocyte chemoattractant protein 1 (MCP-1) in PBC (in the small broken line boxes) is shown (C). Regression analysis (D); this example compares signals generated using the PBC probe with signals generated using a probe derived from non-diseased tissue (the normal liver probe) for each gene on the ATLAS Human Gene Array 1.0. The regression line equation was PBC=2.0684×non-diseased0.83854, r2=0.76, p<0.0001. The extent of differential expression for each gene was determined as a ratio of the raw array signal intensity to the signal intensity calculated from the regression line formula.
Figure 2 .
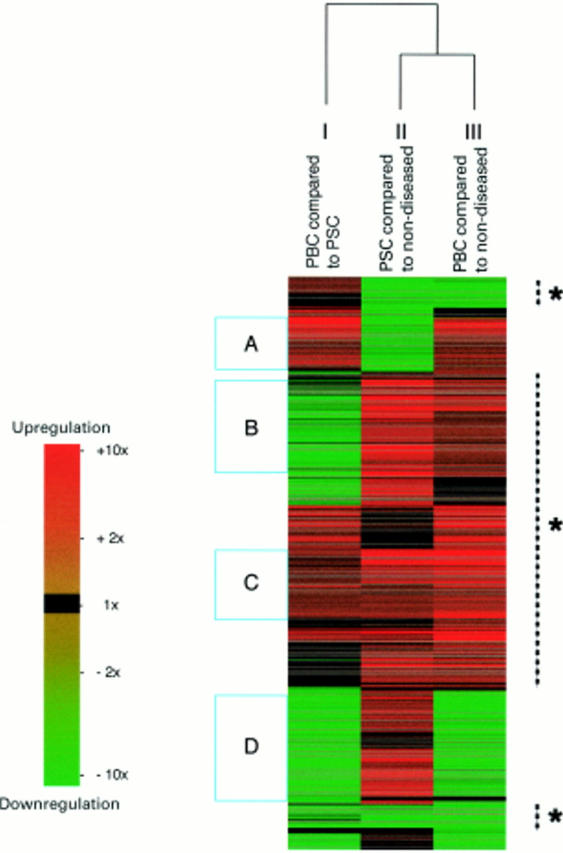
Clustering of the 874 genes. Each line across all comparisons represents a single gene with upregulation indicated in increasing red and downregulation indicated in increasing green. Genes were clustered according to the nature and extent of their differential expression over the three comparisons. There was more than 70% similarity in the patterns of expression in primary biliary cirrhosis (PBC) and primary sclerosing cholangitis (PSC) associated cirrhosis compared with non-diseased tissue (indicated by the broken lines with asterisks). This graphical depiction of the data identifies groups of genes, such as those that had increased expression in: PBC compared with both non-diseased tissue and PSC associated cirrhosis (A); both PBC and PSC associated cirrhosis compared with non-diseased liver (B, C); PBC compared with PSC associated cirrhosis in addition to both of these diseases compared with non-diseased liver (C); and PSC but not PBC compared with non-diseased liver (D).
Figure 3 .
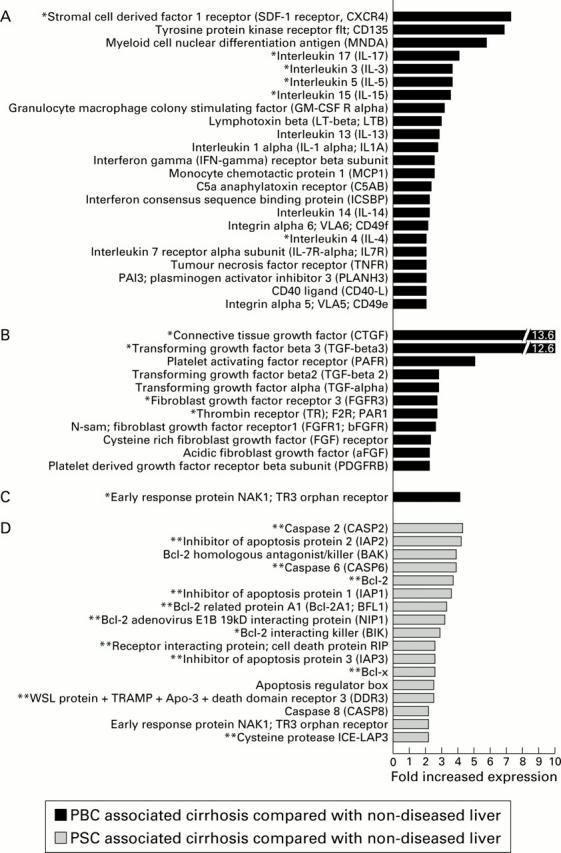
Upregulation of inflammatory, fibrosis, and apoptosis associated genes. Inflammation associated genes (A), fibrosis associated genes (B), and apoptosis associated genes (C) that were upregulated 2.0-fold or greater in primary biliary cirrhosis (PBC) compared with non-diseased liver tissue. Apoptosis associated gene upregulation of 2.0-fold or greater in primary sclerosing cholangitis (PSC) associated cirrhosis compared with non-diseased liver tissue is also shown (D). Genes marked with an asterisk were upregulated greater than twofold in PBC compared with both non-diseased liver tissue and PSC associated cirrhosis (*A-C). Genes marked with two asterisks were upregulated greater than twofold in PSC associated cirrhosis compared with both non-diseased liver tissue and PBC (**D).
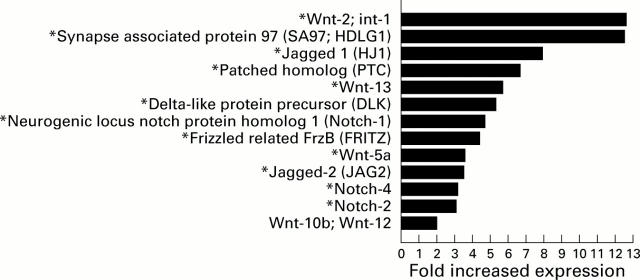
Figure 4 .
Upregulation of Drosophila homologues. Drosophila homologues that were upregulated 2.0-fold or greater in primary biliary cirrhosis (PBC) compared with non-diseased liver tissue. Genes marked with an asterisk were upregulated greater than twofold in PBC compared with both non-diseased liver tissue and primary sclerosing cholangitis associated cirrhosis.
Figure 5 .
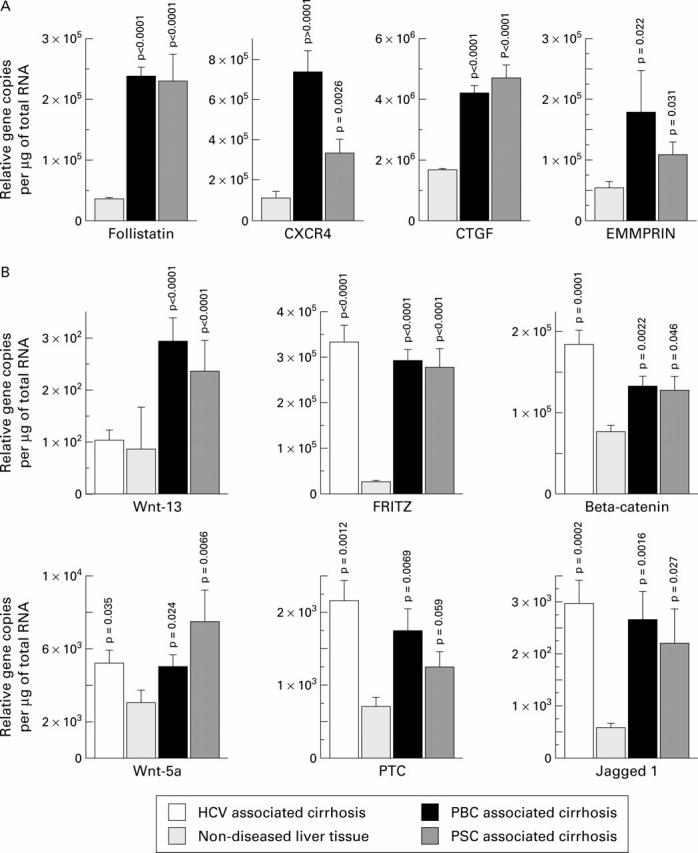
Differential gene expression examined by reverse transcriptase-polymerase chain reaction (RT-PCR). Quantitative real-time RT-PCR data (mean (SEM)) on mRNA from primary biliary cirrhosis (PBC) (n=6), primary sclerosing cholangitis (PSC) associated cirrhosis (n=4), hepatitis C virus (HCV) cirrhosis (n=6), and non-diseased donor liver (n=4). The depicted differential expression data are in two groups: (A) follistatin, CXC chemokine receptor 4 (CXCR4), connective tissue growth factor (CTGF), and extracellular matrix metalloproteinase inducer (EMMPRIN) and (B) Drosophila homologues including Wnt-13, secreted frizzled related protein 3 (FRITZ), beta-catenin, and Jagged-1.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Airaksinen M. S., Titievsky A., Saarma M. GDNF family neurotrophic factor signaling: four masters, one servant? Mol Cell Neurosci. 1999 May;13(5):313–325. doi: 10.1006/mcne.1999.0754. [DOI] [PubMed] [Google Scholar]
- Baer H. U., Friess H., Abou-Shady M., Berberat P., Zimmermann A., Gold L. I., Korc M., Büchler M. W. Transforming growth factor betas and their receptors in human liver cirrhosis. Eur J Gastroenterol Hepatol. 1998 Dec;10(12):1031–1039. doi: 10.1097/00042737-199812000-00009. [DOI] [PubMed] [Google Scholar]
- Barasch J., Yang J., Ware C. B., Taga T., Yoshida K., Erdjument-Bromage H., Tempst P., Parravicini E., Malach S., Aranoff T. Mesenchymal to epithelial conversion in rat metanephros is induced by LIF. Cell. 1999 Nov 12;99(4):377–386. doi: 10.1016/s0092-8674(00)81524-x. [DOI] [PubMed] [Google Scholar]
- Baum H. Mitochondrial antigens, molecular mimicry and autoimmune disease. Biochim Biophys Acta. 1995 May 24;1271(1):111–121. doi: 10.1016/0925-4439(95)00017-x. [DOI] [PubMed] [Google Scholar]
- Berg P. A., Klein R., Röcken M. Cytokines in primary biliary cirrhosis. Semin Liver Dis. 1997 May;17(2):115–123. doi: 10.1055/s-2007-1007189. [DOI] [PubMed] [Google Scholar]
- Biswas C., Zhang Y., DeCastro R., Guo H., Nakamura T., Kataoka H., Nabeshima K. The human tumor cell-derived collagenase stimulatory factor (renamed EMMPRIN) is a member of the immunoglobulin superfamily. Cancer Res. 1995 Jan 15;55(2):434–439. [PubMed] [Google Scholar]
- Boutros M., Mlodzik M. Dishevelled: at the crossroads of divergent intracellular signaling pathways. Mech Dev. 1999 May;83(1-2):27–37. doi: 10.1016/s0925-4773(99)00046-5. [DOI] [PubMed] [Google Scholar]
- Braun A., Appel E., Baruch R., Herz U., Botchkarev V., Paus R., Brodie C., Renz H. Role of nerve growth factor in a mouse model of allergic airway inflammation and asthma. Eur J Immunol. 1998 Oct;28(10):3240–3251. doi: 10.1002/(SICI)1521-4141(199810)28:10<3240::AID-IMMU3240>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Cassiman D., van Pelt J., De Vos R., Van Lommel F., Desmet V., Yap S. H., Roskams T. Synaptophysin: A novel marker for human and rat hepatic stellate cells. Am J Pathol. 1999 Dec;155(6):1831–1839. doi: 10.1016/S0002-9440(10)65501-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristofalo V. J. A DNA chip off the aging block. Nat Med. 2000 May;6(5):507–507. doi: 10.1038/74983. [DOI] [PubMed] [Google Scholar]
- DeCastro R., Zhang Y., Guo H., Kataoka H., Gordon M. K., Toole B. p., Biswas G. Human keratinocytes express EMMPRIN, an extracellular matrix metalloproteinase inducer. J Invest Dermatol. 1996 Jun;106(6):1260–1265. doi: 10.1111/1523-1747.ep12348959. [DOI] [PubMed] [Google Scholar]
- Dierick H., Bejsovec A. Cellular mechanisms of wingless/Wnt signal transduction. Curr Top Dev Biol. 1999;43:153–190. doi: 10.1016/s0070-2153(08)60381-6. [DOI] [PubMed] [Google Scholar]
- Ehrhard P. B., Erb P., Graumann U., Otten U. Expression of nerve growth factor and nerve growth factor receptor tyrosine kinase Trk in activated CD4-positive T-cell clones. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):10984–10988. doi: 10.1073/pnas.90.23.10984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen M. B., Spellman P. T., Brown P. O., Botstein D. Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci U S A. 1998 Dec 8;95(25):14863–14868. doi: 10.1073/pnas.95.25.14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman S. L. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem. 2000 Jan 28;275(4):2247–2250. doi: 10.1074/jbc.275.4.2247. [DOI] [PubMed] [Google Scholar]
- Fussey S. P., Ali S. T., Guest J. R., James O. F., Bassendine M. F., Yeaman S. J. Reactivity of primary biliary cirrhosis sera with Escherichia coli dihydrolipoamide acetyltransferase (E2p): characterization of the main immunogenic region. Proc Natl Acad Sci U S A. 1990 May;87(10):3987–3991. doi: 10.1073/pnas.87.10.3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershwin M. E., Ansari A. A., Mackay I. R., Nakanuma Y., Nishio A., Rowley M. J., Coppel R. L. Primary biliary cirrhosis: an orchestrated immune response against epithelial cells. Immunol Rev. 2000 Apr;174:210–225. doi: 10.1034/j.1600-0528.2002.017402.x. [DOI] [PubMed] [Google Scholar]
- Grotendorst G. R. Connective tissue growth factor: a mediator of TGF-beta action on fibroblasts. Cytokine Growth Factor Rev. 1997 Sep;8(3):171–179. doi: 10.1016/s1359-6101(97)00010-5. [DOI] [PubMed] [Google Scholar]
- Guo H., Zucker S., Gordon M. K., Toole B. P., Biswas C. Stimulation of matrix metalloproteinase production by recombinant extracellular matrix metalloproteinase inducer from transfected Chinese hamster ovary cells. J Biol Chem. 1997 Jan 3;272(1):24–27. [PubMed] [Google Scholar]
- Harada K., Kono N., Tsuneyama K., Nakanuma Y. Cell-kinetic study of proliferating bile ductules in various hepatobiliary diseases. Liver. 1998 Aug;18(4):277–284. doi: 10.1111/j.1600-0676.1998.tb00166.x. [DOI] [PubMed] [Google Scholar]
- Harada K., Van de Water J., Leung P. S., Coppel R. L., Ansari A., Nakanuma Y., Gershwin M. E. In situ nucleic acid hybridization of cytokines in primary biliary cirrhosis: predominance of the Th1 subset. Hepatology. 1997 Apr;25(4):791–796. doi: 10.1002/hep.510250402. [DOI] [PubMed] [Google Scholar]
- Hashimoto E., Lindor K. D., Homburger H. A., Dickson E. R., Czaja A. J., Wiesner R. H., Ludwig J. Immunohistochemical characterization of hepatic lymphocytes in primary biliary cirrhosis in comparison with primary sclerosing cholangitis and autoimmune chronic active hepatitis. Mayo Clin Proc. 1993 Nov;68(11):1049–1055. doi: 10.1016/s0025-6196(12)60897-0. [DOI] [PubMed] [Google Scholar]
- Hlavcák P., Sedláková O., Sedlák J., Hunáková L., Duraj J., Sulíková M., Bízik J., Castronovo V., Chorváth B. Cell surface immunophenotype and gelatinase activity of the human breast carcinoma cell line (MCF-7/6) with functionally defective E-cadherin. Neoplasma. 1999;46(1):12–16. [PubMed] [Google Scholar]
- Itoh K., Sokol S. Y. Graded amounts of Xenopus dishevelled specify discrete anteroposterior cell fates in prospective ectoderm. Mech Dev. 1997 Jan;61(1-2):113–125. doi: 10.1016/s0925-4773(96)00627-2. [DOI] [PubMed] [Google Scholar]
- Jones D. E., Palmer J. M., Yeaman S. J., Kirby J. A., Bassendine M. F. Breakdown of tolerance to pyruvate dehydrogenase complex in experimental autoimmune cholangitis: a mouse model of primary biliary cirrhosis. Hepatology. 1999 Jul;30(1):65–70. doi: 10.1002/hep.510300123. [DOI] [PubMed] [Google Scholar]
- Joplin R., Gershwin M. E. Ductular expression of autoantigens in primary biliary cirrhosis. Semin Liver Dis. 1997 May;17(2):97–103. doi: 10.1055/s-2007-1007187. [DOI] [PubMed] [Google Scholar]
- Joplin R., Hishida T., Tsubouchi H., Daikuhara Y., Ayres R., Neuberger J. M., Strain A. J. Human intrahepatic biliary epithelial cells proliferate in vitro in response to human hepatocyte growth factor. J Clin Invest. 1992 Oct;90(4):1284–1289. doi: 10.1172/JCI115992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joutel A., Tournier-Lasserve E. Notch signalling pathway and human diseases. Semin Cell Dev Biol. 1998 Dec;9(6):619–625. doi: 10.1006/scdb.1998.0261. [DOI] [PubMed] [Google Scholar]
- Kaplan D. R., Miller F. D. Signal transduction by the neurotrophin receptors. Curr Opin Cell Biol. 1997 Apr;9(2):213–221. doi: 10.1016/s0955-0674(97)80065-8. [DOI] [PubMed] [Google Scholar]
- Kogure K., Omata W., Kanzaki M., Zhang Y. Q., Yasuda H., Mine T., Kojima I. A single intraportal administration of follistatin accelerates liver regeneration in partially hepatectomized rats. Gastroenterology. 1995 Apr;108(4):1136–1142. doi: 10.1016/0016-5085(95)90212-0. [DOI] [PubMed] [Google Scholar]
- Kogure K., Zhang Y. Q., Maeshima A., Suzuki K., Kuwano H., Kojima I. The role of activin and transforming growth factor-beta in the regulation of organ mass in the rat liver. Hepatology. 2000 Apr;31(4):916–921. doi: 10.1053/he.2000.6100. [DOI] [PubMed] [Google Scholar]
- Kogure K., Zhang Y. Q., Shibata H., Kojima I. Immediate onset of DNA synthesis in remnant rat liver after 90% hepatectomy by an administration of follistatin. J Hepatol. 1998 Dec;29(6):977–984. doi: 10.1016/s0168-8278(98)80126-8. [DOI] [PubMed] [Google Scholar]
- Krams S. M., Van de Water J., Coppel R. L., Esquivel C., Roberts J., Ansari A., Gershwin M. E. Analysis of hepatic T lymphocyte and immunoglobulin deposits in patients with primary biliary cirrhosis. Hepatology. 1990 Aug;12(2):306–313. doi: 10.1002/hep.1840120219. [DOI] [PubMed] [Google Scholar]
- Lee Y. J., Swencki B., Shoichet S., Shivdasani R. A. A possible role for the high mobility group box transcription factor Tcf-4 in vertebrate gut epithelial cell differentiation. J Biol Chem. 1999 Jan 15;274(3):1566–1572. doi: 10.1074/jbc.274.3.1566. [DOI] [PubMed] [Google Scholar]
- Levy M. T., McCaughan G. W., Abbott C. A., Park J. E., Cunningham A. M., Müller E., Rettig W. J., Gorrell M. D. Fibroblast activation protein: a cell surface dipeptidyl peptidase and gelatinase expressed by stellate cells at the tissue remodelling interface in human cirrhosis. Hepatology. 1999 Jun;29(6):1768–1778. doi: 10.1002/hep.510290631. [DOI] [PubMed] [Google Scholar]
- Li L., Krantz I. D., Deng Y., Genin A., Banta A. B., Collins C. C., Qi M., Trask B. J., Kuo W. L., Cochran J. Alagille syndrome is caused by mutations in human Jagged1, which encodes a ligand for Notch1. Nat Genet. 1997 Jul;16(3):243–251. doi: 10.1038/ng0797-243. [DOI] [PubMed] [Google Scholar]
- Lim M., Martinez T., Jablons D., Cameron R., Guo H., Toole B., Li J. D., Basbaum C. Tumor-derived EMMPRIN (extracellular matrix metalloproteinase inducer) stimulates collagenase transcription through MAPK p38. FEBS Lett. 1998 Dec 11;441(1):88–92. doi: 10.1016/s0014-5793(98)01474-4. [DOI] [PubMed] [Google Scholar]
- Louis A. A., Van Eyken P., Haber B. A., Hicks C., Weinmaster G., Taub R., Rand E. B. Hepatic jagged1 expression studies. Hepatology. 1999 Nov;30(5):1269–1275. doi: 10.1002/hep.510300512. [DOI] [PubMed] [Google Scholar]
- Mackay I. R., Whittingham S., Fida S., Myers M., Ikuno N., Gershwin M. E., Rowley M. J. The peculiar autoimmunity of primary biliary cirrhosis. Immunol Rev. 2000 Apr;174:226–237. doi: 10.1034/j.1600-0528.2002.017410.x. [DOI] [PubMed] [Google Scholar]
- Marra F., DeFranco R., Grappone C., Milani S., Pastacaldi S., Pinzani M., Romanelli R. G., Laffi G., Gentilini P. Increased expression of monocyte chemotactic protein-1 during active hepatic fibrogenesis: correlation with monocyte infiltration. Am J Pathol. 1998 Feb;152(2):423–430. [PMC free article] [PubMed] [Google Scholar]
- Matsumoto K., Fujii H., Michalopoulos G., Fung J. J., Demetris A. J. Human biliary epithelial cells secrete and respond to cytokines and hepatocyte growth factors in vitro: interleukin-6, hepatocyte growth factor and epidermal growth factor promote DNA synthesis in vitro. Hepatology. 1994 Aug;20(2):376–382. [PubMed] [Google Scholar]
- McCaughan G. W., Gorrell M. D., Bishop G. A., Abbott C. A., Shackel N. A., McGuinness P. H., Levy M. T., Sharland A. F., Bowen D. G., Yu D. Molecular pathogenesis of liver disease: an approach to hepatic inflammation, cirrhosis and liver transplant tolerance. Immunol Rev. 2000 Apr;174:172–191. doi: 10.1034/j.1600-0528.2002.017420.x. [DOI] [PubMed] [Google Scholar]
- Merino R., Macias D., Gañan Y., Rodriguez-Leon J., Economides A. N., Rodriguez-Esteban C., Izpisua-Belmonte J. C., Hurle J. M. Control of digit formation by activin signalling. Development. 1999 May;126(10):2161–2170. doi: 10.1242/dev.126.10.2161. [DOI] [PubMed] [Google Scholar]
- Messing A. Nestin in the liver--lessons from the brain. Hepatology. 1999 Feb;29(2):602–603. doi: 10.1002/hep.510290234. [DOI] [PubMed] [Google Scholar]
- Milani S., Herbst H., Schuppan D., Stein H., Surrenti C. Transforming growth factors beta 1 and beta 2 are differentially expressed in fibrotic liver disease. Am J Pathol. 1991 Dec;139(6):1221–1229. [PMC free article] [PubMed] [Google Scholar]
- Morrison T. B., Weis J. J., Wittwer C. T. Quantification of low-copy transcripts by continuous SYBR Green I monitoring during amplification. Biotechniques. 1998 Jun;24(6):954-8, 960, 962. [PubMed] [Google Scholar]
- Napoli J., Bishop G. A., McCaughan G. W. Increased intrahepatic messenger RNA expression of interleukins 2, 6, and 8 in human cirrhosis. Gastroenterology. 1994 Sep;107(3):789–798. doi: 10.1016/0016-5085(94)90128-7. [DOI] [PubMed] [Google Scholar]
- Niki T., Pekny M., Hellemans K., Bleser P. D., Berg K. V., Vaeyens F., Quartier E., Schuit F., Geerts A. Class VI intermediate filament protein nestin is induced during activation of rat hepatic stellate cells. Hepatology. 1999 Feb;29(2):520–527. doi: 10.1002/hep.510290232. [DOI] [PubMed] [Google Scholar]
- Palmer J. M., Diamond A. G., Yeaman S. J., Bassendine M. F., Jones D. E. T cell responses to the putative dominant autoepitope in primary biliary cirrhosis (PBC). Clin Exp Immunol. 1999 Apr;116(1):133–139. doi: 10.1046/j.1365-2249.1999.00803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradis V., Dargere D., Vidaud M., De Gouville A. C., Huet S., Martinez V., Gauthier J. M., Ba N., Sobesky R., Ratziu V. Expression of connective tissue growth factor in experimental rat and human liver fibrosis. Hepatology. 1999 Oct;30(4):968–976. doi: 10.1002/hep.510300425. [DOI] [PubMed] [Google Scholar]
- Phillips D. J., de Kretser D. M. Follistatin: a multifunctional regulatory protein. Front Neuroendocrinol. 1998 Oct;19(4):287–322. doi: 10.1006/frne.1998.0169. [DOI] [PubMed] [Google Scholar]
- Robey E., Chang D., Itano A., Cado D., Alexander H., Lans D., Weinmaster G., Salmon P. An activated form of Notch influences the choice between CD4 and CD8 T cell lineages. Cell. 1996 Nov 1;87(3):483–492. doi: 10.1016/s0092-8674(00)81368-9. [DOI] [PubMed] [Google Scholar]
- Russell C. E., Hedger M. P., Brauman J. N., de Kretser D. M., Phillips D. J. Activin A regulates growth and acute phase proteins in the human liver cell line, HepG2. Mol Cell Endocrinol. 1999 Feb 25;148(1-2):129–136. doi: 10.1016/s0303-7207(98)00226-3. [DOI] [PubMed] [Google Scholar]
- Santos R. M., Norton P., Degli Esposti S., Zern M. A. TGF-beta isoforms in alcoholic liver disease. J Gastroenterol. 1998 Jun;33(3):383–389. doi: 10.1007/s005350050100. [DOI] [PubMed] [Google Scholar]
- Schwall R. H., Robbins K., Jardieu P., Chang L., Lai C., Terrell T. G. Activin induces cell death in hepatocytes in vivo and in vitro. Hepatology. 1993 Aug;18(2):347–356. doi: 10.1016/0270-9139(93)90018-i. [DOI] [PubMed] [Google Scholar]
- Sehgal A., Boynton A. L., Young R. F., Vermeulen S. S., Yonemura K. S., Kohler E. P., Aldape H. C., Simrell C. R., Murphy G. P. Application of the differential hybridization of Atlas Human expression arrays technique in the identification of differentially expressed genes in human glioblastoma multiforme tumor tissue. J Surg Oncol. 1998 Apr;67(4):234–241. doi: 10.1002/(sici)1096-9098(199804)67:4<234::aid-jso5>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Sen M., Lauterbach K., El-Gabalawy H., Firestein G. S., Corr M., Carson D. A. Expression and function of wingless and frizzled homologs in rheumatoid arthritis. Proc Natl Acad Sci U S A. 2000 Mar 14;97(6):2791–2796. doi: 10.1073/pnas.050574297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields P. L., Morland C. M., Salmon M., Qin S., Hubscher S. G., Adams D. H. Chemokine and chemokine receptor interactions provide a mechanism for selective T cell recruitment to specific liver compartments within hepatitis C-infected liver. J Immunol. 1999 Dec 1;163(11):6236–6243. [PubMed] [Google Scholar]
- Shim C., Zhang W., Rhee C. H., Lee J. H. Profiling of differentially expressed genes in human primary cervical cancer by complementary DNA expression array. Clin Cancer Res. 1998 Dec;4(12):3045–3050. [PubMed] [Google Scholar]
- Takahashi M., Asai N., Iwashita T., Murakami H., Ito S. Mechanisms of development of multiple endocrine neoplasia type 2 and Hirschsprung's disease by ret mutations. Recent Results Cancer Res. 1998;154:229–236. doi: 10.1007/978-3-642-46870-4_14. [DOI] [PubMed] [Google Scholar]
- Teoh K. L., Mackay I. R., Rowley M. J., Fussey S. P. Enzyme inhibitory autoantibodies to pyruvate dehydrogenase complex in primary biliary cirrhosis differ for mammalian, yeast and bacterial enzymes: implications for molecular mimicry. Hepatology. 1994 Apr;19(4):1029–1033. [PubMed] [Google Scholar]
- Tjandra K., Sharkey K. A., Swain M. G. Progressive development of a Th1-type hepatic cytokine profile in rats with experimental cholangitis. Hepatology. 2000 Feb;31(2):280–290. doi: 10.1002/hep.510310204. [DOI] [PubMed] [Google Scholar]
- Torcia M., Bracci-Laudiero L., Lucibello M., Nencioni L., Labardi D., Rubartelli A., Cozzolino F., Aloe L., Garaci E. Nerve growth factor is an autocrine survival factor for memory B lymphocytes. Cell. 1996 May 3;85(3):345–356. doi: 10.1016/s0092-8674(00)81113-7. [DOI] [PubMed] [Google Scholar]
- Trim N., Morgan S., Evans M., Issa R., Fine D., Afford S., Wilkins B., Iredale J. Hepatic stellate cells express the low affinity nerve growth factor receptor p75 and undergo apoptosis in response to nerve growth factor stimulation. Am J Pathol. 2000 Apr;156(4):1235–1243. doi: 10.1016/S0002-9440(10)64994-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura H., Chang C. Antisense TR3 orphan receptor can increase prostate cancer cell viability with etoposide treatment. Endocrinology. 1998 May;139(5):2329–2334. doi: 10.1210/endo.139.5.5969. [DOI] [PubMed] [Google Scholar]
- Van Den Berg D. J., Sharma A. K., Bruno E., Hoffman R. Role of members of the Wnt gene family in human hematopoiesis. Blood. 1998 Nov 1;92(9):3189–3202. [PubMed] [Google Scholar]
- Van de Water J., Ansari A. A., Surh C. D., Coppel R., Roche T., Bonkovsky H., Kaplan M., Gershwin M. E. Evidence for the targeting by 2-oxo-dehydrogenase enzymes in the T cell response of primary biliary cirrhosis. J Immunol. 1991 Jan 1;146(1):89–94. [PubMed] [Google Scholar]
- Van de Water J., Ansari A., Prindiville T., Coppel R. L., Ricalton N., Kotzin B. L., Liu S., Roche T. E., Krams S. M., Munoz S. Heterogeneity of autoreactive T cell clones specific for the E2 component of the pyruvate dehydrogenase complex in primary biliary cirrhosis. J Exp Med. 1995 Feb 1;181(2):723–733. doi: 10.1084/jem.181.2.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Water J., Turchany J., Leung P. S., Lake J., Munoz S., Surh C. D., Coppel R., Ansari A., Nakanuma Y., Gershwin M. E. Molecular mimicry in primary biliary cirrhosis. Evidence for biliary epithelial expression of a molecule cross-reactive with pyruvate dehydrogenase complex-E2. J Clin Invest. 1993 Jun;91(6):2653–2664. doi: 10.1172/JCI116504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang E. H., Tjian R. Promoter-selective transcriptional defect in cell cycle mutant ts13 rescued by hTAFII250. Science. 1994 Feb 11;263(5148):811–814. doi: 10.1126/science.8303298. [DOI] [PubMed] [Google Scholar]
- Wang E. H., Zou S., Tjian R. TAFII250-dependent transcription of cyclin A is directed by ATF activator proteins. Genes Dev. 1997 Oct 15;11(20):2658–2669. doi: 10.1101/gad.11.20.2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washburn T., Schweighoffer E., Gridley T., Chang D., Fowlkes B. J., Cado D., Robey E. Notch activity influences the alphabeta versus gammadelta T cell lineage decision. Cell. 1997 Mar 21;88(6):833–843. doi: 10.1016/s0092-8674(00)81929-7. [DOI] [PubMed] [Google Scholar]
- Yasoshima M., Nakanuma Y., Tsuneyama K., Van de Water J., Gershwin M. E. Immunohistochemical analysis of adhesion molecules in the micro-environment of portal tracts in relation to aberrant expression of PDC-E2 and HLA-DR on the bile ducts in primary biliary cirrhosis. J Pathol. 1995 Mar;175(3):319–325. doi: 10.1002/path.1711750310. [DOI] [PubMed] [Google Scholar]
- Yasuda H., Mine T., Shibata H., Eto Y., Hasegawa Y., Takeuchi T., Asano S., Kojima I. Activin A: an autocrine inhibitor of initiation of DNA synthesis in rat hepatocytes. J Clin Invest. 1993 Sep;92(3):1491–1496. doi: 10.1172/JCI116727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeaman S. J., Kirby J. A., Jones D. E. Autoreactive responses to pyruvate dehydrogenase complex in the pathogenesis of primary biliary cirrhosis. Immunol Rev. 2000 Apr;174:238–249. doi: 10.1034/j.1600-0528.2002.00021h.x. [DOI] [PubMed] [Google Scholar]
- Zhang Y. Q., Kanzaki M., Shibata H., Kojima I. Regulation of the expression of follistatin in rat hepatocytes. Biochim Biophys Acta. 1997 Nov 20;1354(3):204–210. doi: 10.1016/s0167-4781(97)00085-7. [DOI] [PubMed] [Google Scholar]
- van Weering D. H., Bos J. L. Signal transduction by the receptor tyrosine kinase Ret. Recent Results Cancer Res. 1998;154:271–281. doi: 10.1007/978-3-642-46870-4_18. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.