Abstract
BACKGROUND—Gall bladder functions are modulated by neurones intrinsic to the organ. Data are available on the neurochemical composition of intrinsic and extrinsic nerves innervating the gall bladder but are lacking on specific functional classes of gall bladder neurones. AIMS—To characterise the intrinsic motor neurones of the gall bladder and identify their roles using pharmacological techniques. METHODS—Retrograde tracing from the possum gall bladder muscle in vitro allowed identification of intrinsic motor neurones. Subsequently, their content of choline acetyltransferase and nitric oxide synthase, markers of acetylcholine and nitric oxide containing neurones, was established using immunohistochemical techniques. Organ bath pharmacology was used to evaluate neurotransmission by acetylcholine and nitric oxide in gall bladder muscle strips. RESULTS—Innervation of the gall bladder musculature by neurones of both the muscular and serosal plexuses was demonstrated. A large proportion (62%) of these motor neurones were immunoreactive for nitric oxide synthase. All gall bladder neurones showed immunoreactivity for choline acetyltransferase. Organ bath pharmacology confirmed the neuroanatomical data, showing acetylcholine and nitric oxide mediating neurotransmission to the gall bladder musculature. CONCLUSIONS—Neurones containing acetylcholine and nitric oxide, located within the muscular and serosal plexuses, provide excitatory and inhibitory motor innervation of the gall bladder, respectively. The large inhibitory innervation suggests active relaxation of the gall bladder during filling, mediated by intrinsic nerves. Keywords: excitatory/inhibitory neurotransmission; gall bladder; motility; nitric oxide; acetylcholine; possum
Full Text
The Full Text of this article is available as a PDF (197.2 KB).
Figure 1 .
Photomicrographs of a ganglion of the muscular plexus in the possum gall bladder. All three neurones show choline acetyltransferase (ChAT) immunoreactivity (A). A neurone from the same ganglion shows nitric oxide synthase (NOS) immunoreactivity (arrow) while the others are immunonegative for NOS (arrowheads) (B). Bar, 20 µm.
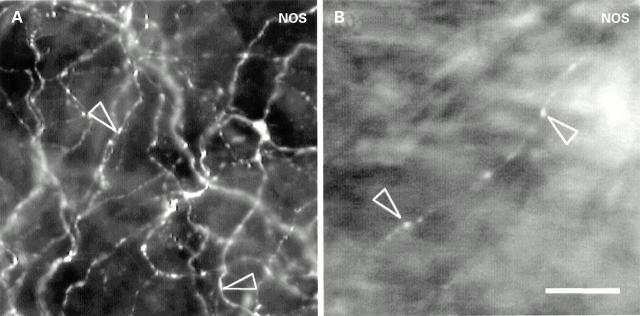
Figure 2 .
Photomicrograph of nitric oxide synthase (NOS) immunoreactive nerve fibres within the possum gall bladder. NOS immunoreactive nerve fibres (arrowheads) were present within the non-ganglionated subepithelial plexus (A) and the muscle (B) of the gall bladder. Bar, 20 µm.
Figure 3 .
Retrogradely labelled neurones were found within the muscular (A) and serosal plexuses (C) on 1'-didodecyl-3,3,3'3'-tetra-methyl-indocarbocyanine perchlorate (DiI) application to the gall bladder muscle. Some DiI labelled cells showed immunoreactivity for nitric oxide synthase (NOS) (arrows) while others were immunonegative (arrowhead) (B, D). Bar, 20 µm.
Figure 4 .
(A) Scatterplots of neurones retrogradely labelled on 1'-didodecyl-3,3,3'3'-tetra-methyl-indocarbocyanine perchlorate (DiI) application to the gall bladder muscle in a representative preparation. The DiI labelled neurones were primarily located close to the application site (all within 10 mm). (B) Cumulative data of all DiI traced neurones from five preparations showed that nitric oxide synthase (NOS) immunoreactive neurones within both the muscular and serosal plexus were polarised in their distribution along the longitudinal axis of the gall bladder, projecting predominantly from the neck towards the apex, but NOS immunonegative cells did not show a polarised distribution.
Figure 5 .
Representative traces and cumulative data of gall bladder muscle strip pharmacology. Electrical field stimulation EFS (arrows) produced consistent gall bladder contraction under control conditions (A). Under non-adrenergic non-cholinergic (NANC) conditions, EFS produced relaxation (B). This response was abolished by N omega-nitro-L-arginine methyl ester (L-NAME) (C). Cumulative data (D) are expressed as mean (SEM). All responses differed significantly (*p<0.05) from each other. The broken lines and double headed arrows illustrate the parameters used for quantifying the EFS induced contractions (A) and relaxations (B).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abiru H., Sarna S. K., Condon R. E. Contractile mechanisms of gallbladder filling and emptying in dogs. Gastroenterology. 1994 Jun;106(6):1652–1661. doi: 10.1016/0016-5085(94)90423-5. [DOI] [PubMed] [Google Scholar]
- Brookes S. J., Hennig G., Schemann M. Identification of motor neurons to the circular muscle of the guinea pig gastric corpus. J Comp Neurol. 1998 Jul 27;397(2):268–280. doi: 10.1002/(sici)1096-9861(19980727)397:2<268::aid-cne8>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Brookes S. J., Steele P. A., Costa M. Identification and immunohistochemistry of cholinergic and non-cholinergic circular muscle motor neurons in the guinea-pig small intestine. Neuroscience. 1991;42(3):863–878. doi: 10.1016/0306-4522(91)90050-x. [DOI] [PubMed] [Google Scholar]
- Carbone A., Schloithe A. C., Harvey J. R., Baker R. A., Saccone G. T. Gastrin-releasing peptide stimulates possum gallbladder contractility in vitro. Peptides. 1997;18(7):1067–1071. doi: 10.1016/s0196-9781(97)00034-x. [DOI] [PubMed] [Google Scholar]
- De Giorgio R., Zittel T. T., Parodi J. E., Becker J. M., Brunicardi F. C., Go V. L., Brecha N. C., Sternini C. Peptide immunoreactivities in the ganglionated plexuses and nerve fibers innervating the human gallbladder. J Auton Nerv Syst. 1995 Jan 20;51(1):37–47. doi: 10.1016/0165-1838(95)80005-u. [DOI] [PubMed] [Google Scholar]
- Gonda T., Akiyoshi H., Ichihara K. Hyperplastic innervation of vasoactive intestinal peptide in human gallbladder with cholelithiasis. Histol Histopathol. 1995 Jul;10(3):669–672. [PubMed] [Google Scholar]
- Gonda T., Akiyoshi H., Ichihara K. Scanning electron microscopic observations of nerves in the guinea-pig gallbladder after an acetylcholinesterase histochemistry. J Smooth Muscle Res. 1995 Aug;31(4):153–162. doi: 10.1540/jsmr.31.153. [DOI] [PubMed] [Google Scholar]
- Greaves R., Miller J., O'Donnell L., McLean A., Farthing M. J. Effect of the nitric oxide donor, glyceryl trinitrate, on human gall bladder motility. Gut. 1998 Mar;42(3):410–413. doi: 10.1136/gut.42.3.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoursheed M., Krajci D., Oriowo M. A., Kadavil E., Philip E. K., Thulesius O. Neurogenic control of the ovine gallbladder: ultrastructural and functional study. Digestion. 1998 Jul-Aug;59(4):335–342. doi: 10.1159/000007512. [DOI] [PubMed] [Google Scholar]
- Mawe G. M., Gershon M. D. Structure, afferent innervation, and transmitter content of ganglia of the guinea pig gallbladder: relationship to the enteric nervous system. J Comp Neurol. 1989 May 15;283(3):374–390. doi: 10.1002/cne.902830306. [DOI] [PubMed] [Google Scholar]
- Mawe G. M., Talmage E. K., Cornbrooks E. B., Gokin A. P., Zhang L., Jennings L. J. Innervation of the gallbladder: structure, neurochemical coding, and physiological properties of guinea pig gallbladder ganglia. Microsc Res Tech. 1997 Oct 1;39(1):1–13. doi: 10.1002/(SICI)1097-0029(19971001)39:1<1::AID-JEMT1>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- McKirdy M. L., McKirdy H. C., Johnson C. D. Non-adrenergic non-cholinergic inhibitory innervation shown by electrical field stimulation of isolated strips of human gall bladder muscle. Gut. 1994 Mar;35(3):412–416. doi: 10.1136/gut.35.3.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meedeniya A. C., Brookes S. J., Hennig G. W., Costa M. The projections of 5-hydroxytryptamine-accumulating neurones in the myenteric plexus of the small intestine of the guinea-pig. Cell Tissue Res. 1998 Mar;291(3):375–384. doi: 10.1007/s004410051007. [DOI] [PubMed] [Google Scholar]
- Padbury R. T., Baker R. A., Messenger J. P., Toouli J., Furness J. B. Structure and innervation of the extrahepatic biliary system in the Australian possum, Trichosurus vulpecula. HPB Surg. 1993;7(2):125–140. doi: 10.1155/1993/72946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkman H. P., James A. N., Bogar L. J., Bartula L. L., Thomas R. M., Ryan J. P., Myers S. I. Effect of acalculous cholecystitis on gallbladder neuromuscular transmission and contractility. J Surg Res. 2000 Feb;88(2):186–192. doi: 10.1006/jsre.1999.5788. [DOI] [PubMed] [Google Scholar]
- Parkman H. P., Pagano A. P., Martin J. S., Ryan J. P. Electric field stimulation-induced guinea pig gallbladder contractions: role of calcium channels in acetylcholine release. Dig Dis Sci. 1997 Sep;42(9):1919–1925. doi: 10.1023/a:1018819411992. [DOI] [PubMed] [Google Scholar]
- Petersen K. U., Goergen R., Höfken F., Macherey H. J., Sprakties G. Electrogenic bicarbonate secretion in gallbladder: induction by barium via neuronal, possibly VIP-ergic pathways. Naunyn Schmiedebergs Arch Pharmacol. 1993 Nov;348(5):526–535. doi: 10.1007/BF00173214. [DOI] [PubMed] [Google Scholar]
- Pfannkuche H., Reiche D., Firzlaff U., Sann H., Schemann M. Enkephalin-immunoreactive subpopulations in the myenteric plexus of the guinea-pig fundus project primarily to the muscle and not to the mucosa. Cell Tissue Res. 1998 Oct;294(1):45–55. doi: 10.1007/s004410051155. [DOI] [PubMed] [Google Scholar]
- Porter A. J., Wattchow D. A., Brookes S. J., Costa M. The neurochemical coding and projections of circular muscle motor neurons in the human colon. Gastroenterology. 1997 Dec;113(6):1916–1923. doi: 10.1016/s0016-5085(97)70011-8. [DOI] [PubMed] [Google Scholar]
- Simula M. E., Brookes S. J., Meedeniya A. C., Toouli J., Saccone G. T. Distribution of nitric oxide synthase and vasoactive intestinal polypeptide immunoreactivity in the sphincter of Oddi and duodenum of the possum. Cell Tissue Res. 2001 Apr;304(1):31–41. doi: 10.1007/s004410100357. [DOI] [PubMed] [Google Scholar]
- Sänger P. R., Sommerfeldt D. W., Hanisch E. W. Non-adrenergic, non-cholinergic regulation of stone-diseased and stone-free human gallbladders. Eur J Gastroenterol Hepatol. 1999 Oct;11(10):1085–1091. doi: 10.1097/00042737-199910000-00003. [DOI] [PubMed] [Google Scholar]
- Talmage E. K., Pouliot W. A., Schemann M., Mawe G. M. Structure and chemical coding of human, canine and opossum gallbladder ganglia. Cell Tissue Res. 1996 May;284(2):289–302. doi: 10.1007/s004410050589. [DOI] [PubMed] [Google Scholar]
- Toouli J., Bushell M., Stevenson G., Dent J., Wycherley A., Iannos J. Gallbladder emptying in man related to fasting duodenal migrating motor contractions. Aust N Z J Surg. 1986 Feb;56(2):147–151. doi: 10.1111/j.1445-2197.1986.tb01872.x. [DOI] [PubMed] [Google Scholar]
- Uemura S., Pompolo S., Furness J. B., Hardy K. J. Nitric oxide synthase in neurons of the human gall-bladder and its colocalization with neuropeptides. J Gastroenterol Hepatol. 1997 Mar;12(3):257–265. doi: 10.1111/j.1440-1746.1997.tb00418.x. [DOI] [PubMed] [Google Scholar]