Abstract
BACKGROUND—South African Helicobacter pylori isolates are characterised by the universal presence of cagA but have differences in vacuolating cytotoxin gene (vacA) alleles which correlate with clinically significant disease. However, the candidate virulence marker gene iceA has not been investigated. AIM—To characterise the genetic organisation and heterogeneity of iceA genotypes in different South African clinical isolates. PATIENTS AND METHODS—We studied H pylori strains isolated from 86 dyspeptic patients (30 with peptic ulcer disease (PUD), 19 with distal gastric adenocarcinoma (GC), and 37 with non-erosive gastritis) for the presence of iceA1 or iceA2 genes, and for differences in the genetic organisation of iceA2 by polymerase chain reaction, Southern hybridisation analysis, and sequencing. RESULTS—Genetic analysis of iceA1 demonstrated significant homology (92-95%) with the USA type strain 26695 and probably functions as a transcriptional regulator, while a novel variant (iceA2D') of iceA2 and marked differences in predicted protein secondary structure of the iceA2 protein were defined. iceA1 was detected in 68% and iceA2 in 80% of all clinical isolates. Although approximately 40% of patients had both strains, a higher prevalence (p< 0.01) of GC patients were infected with iceA1 isolates which were invariably vacA s1/iceA1 (p< 0.005 v gastritis). Isolates from PUD patients were distinguished by the structurally altered iceA2D variant (53%; p<0.03 v gastritis) while the iceA2C variant distinguished isolates from patients with gastritis alone (67%; p< 0.005 v PUD). CONCLUSION—In this study, an association between iceA1 and GC was noted while differences in variants of iceA2 differentiated between PUD and gastritis alone. Combination analyses of iceA genotypes and vacA alleles supported these associations. Keywords: adenocarcinoma; gastritis; Helicobacter pylori; iceA; peptic ulceration; protein prediction; sequencing; South Africa
Full Text
The Full Text of this article is available as a PDF (227.7 KB).
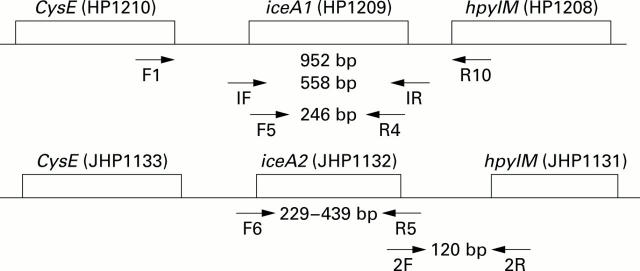
Figure 1 .
Primers, sites, and amplicon sizes for iceA1 (HP1209, GenBank Accession No AE000511) and iceA2 (JHP1132, GenBank Accession No AE001541) (see text for details).
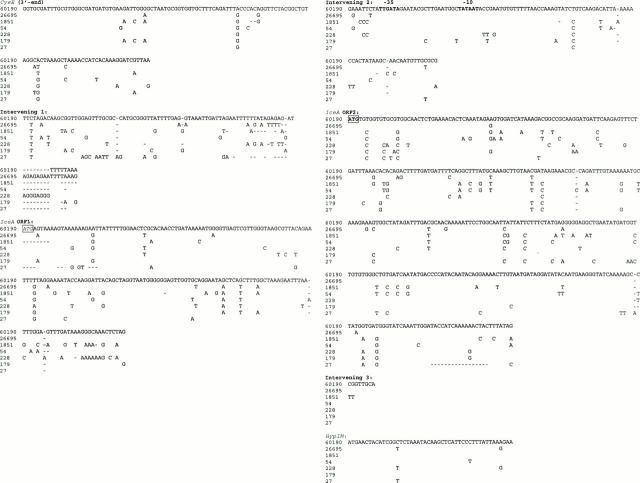
Figure 2 .
Alignment of nucleotide sequences of 60190, 26695, and five South African strains showing the 3' end of cysE, the complete iceA1 gene (ORF1 and ORF2), intervening sequences (1-3), and the 5' end of hypIM putative start codon. ATG617 (italicised and boxed) is the start codon for nlaIIIR and is only conserved in 3/5 strains. None of the strains however is predicted to produce the NLAIII homologue of 228 amino acids. ATG919 (boxed/bold), the start codon for iceA1, is conserved in all five South African isolates, as are the upstream transcription boxes (at -35 and -10). Predicted iceA1 proteins range in size from 128 to 136 amino acids, except for strain 54 (frameshift results in a protein of 53 amino acids). All isolates shared substantial protein homology (93-96%) with HP1209. Sequence gaps are indicated by dashes (-).
Figure 3 .
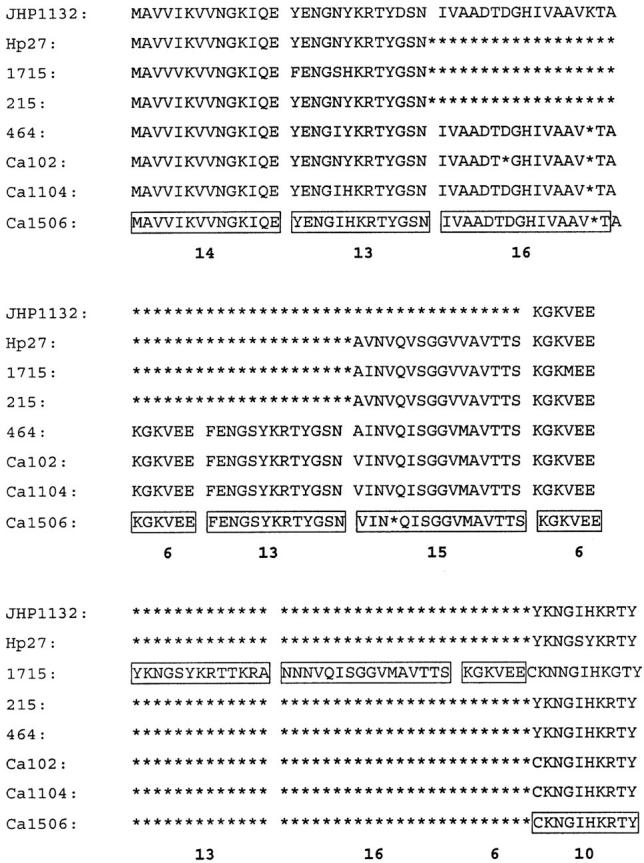
Amino acid alignment of iceA2 genotypes from seven South African isolates and homology with JHP1132. The different peptide domains are boxed and the amino acid number indicated. Asterisks (*) represent gaps in sequence homology.
Figure 4 .
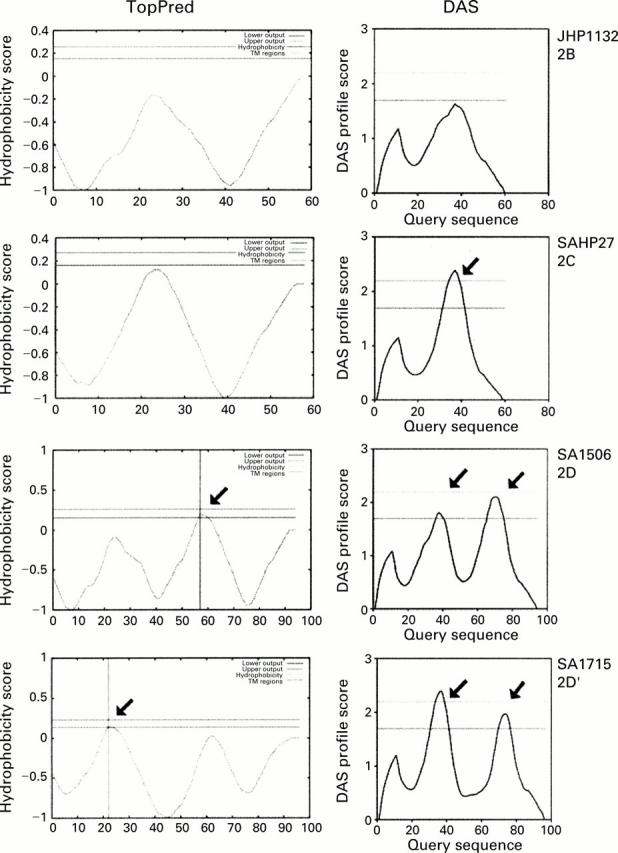
Topology profiles of four iceA2 variants. Left: protein predictions from the TopPred program; right: predictions from the dense alignment sequence (DAS) program. The type strain JHP1132 (iceA2B variant) is indicated in the top panel and South African strains are included with the iceA2 variants signified (2C-2D'). Arrows indicate putative transmembrane regions.
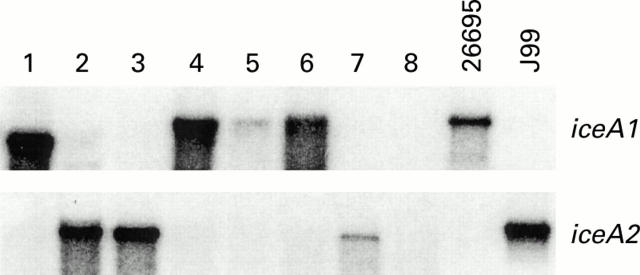
Figure 5 .
Southern analysis of putatively clonal isolates derived from eight parent strains (lanes 1-8) that were originally iceA1+/2+ by polymerase chain reaction. Four were iceA1+, three iceA2+, and one had no hybridisation. Positive controls (lanes 9 and 10) were 26695 (iceA1+) and J99 (iceA2+).
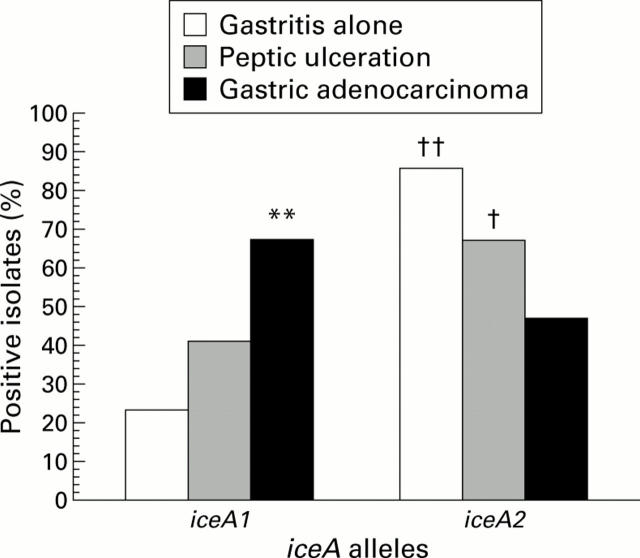
Figure 6 .
Distribution of iceA genotypes in all isolates. **p<0.01 versus gastritis alone; †p<0.04, ††p<0.003 versus gastric cancer.
Figure 7 .
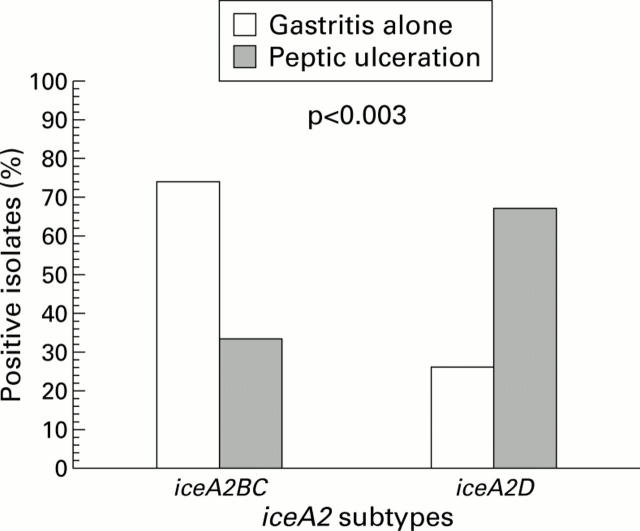
Distribution of iceA2 subtypes in patients with gastritis alone or peptic ulcer disease.
Figure 8 .
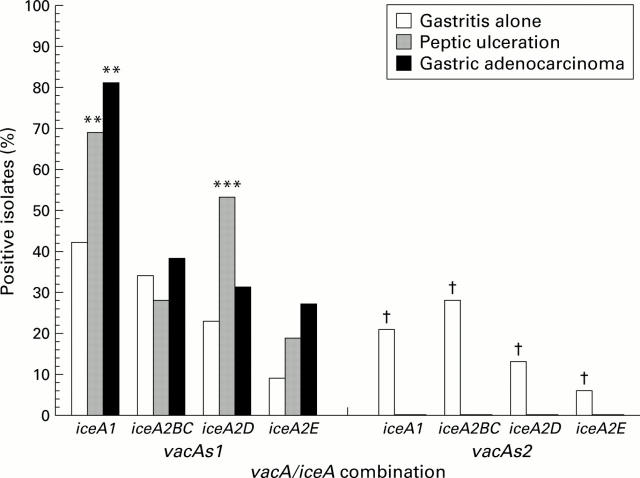
Combination analysis of vacA/iceA alleles and disease outcome in all clinical isolates. **p<0.01, ***p<0.005 versus gastritis alone; †p<0.05 versus peptic ulceration or gastric adenocarcinoma.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alm R. A., Ling L. S., Moir D. T., King B. L., Brown E. D., Doig P. C., Smith D. R., Noonan B., Guild B. C., deJonge B. L. Genomic-sequence comparison of two unrelated isolates of the human gastric pathogen Helicobacter pylori. Nature. 1999 Jan 14;397(6715):176–180. doi: 10.1038/16495. [DOI] [PubMed] [Google Scholar]
- Atherton J. C. The clinical relevance of strain types of Helicobacter pylori. Gut. 1997 Jun;40(6):701–703. doi: 10.1136/gut.40.6.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cserzö M., Wallin E., Simon I., von Heijne G., Elofsson A. Prediction of transmembrane alpha-helices in prokaryotic membrane proteins: the dense alignment surface method. Protein Eng. 1997 Jun;10(6):673–676. doi: 10.1093/protein/10.6.673. [DOI] [PubMed] [Google Scholar]
- Cuff J. A., Barton G. J. Evaluation and improvement of multiple sequence methods for protein secondary structure prediction. Proteins. 1999 Mar 1;34(4):508–519. doi: 10.1002/(sici)1097-0134(19990301)34:4<508::aid-prot10>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Donahue J. P., Peek R. M., Van Doorn L. J., Thompson S. A., Xu Q., Blaser M. J., Miller G. G. Analysis of iceA1 transcription in Helicobacter pylori. Helicobacter. 2000 Mar;5(1):1–12. doi: 10.1046/j.1523-5378.2000.00008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois A., Berg D. E., Incecik E. T., Fiala N., Heman-Ackah L. M., Perez-Perez G. I., Blaser M. J. Transient and persistent experimental infection of nonhuman primates with Helicobacter pylori: implications for human disease. Infect Immun. 1996 Aug;64(8):2885–2891. doi: 10.1128/iai.64.8.2885-2891.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueiredo C., Quint W. G., Sanna R., Sablon E., Donahue J. P., Xu Q., Miller G. G., Peek R. M., Jr, Blaser M. J., van Doorn L. J. Genetic organization and heterogeneity of the iceA locus of Helicobacter pylori. Gene. 2000 Apr 4;246(1-2):59–68. doi: 10.1016/s0378-1119(00)00054-8. [DOI] [PubMed] [Google Scholar]
- Kidd M., Lastovica A. J., Atherton J. C., Louw J. A. Heterogeneity in the Helicobacter pylori vacA and cagA genes: association with gastroduodenal disease in South Africa? Gut. 1999 Oct;45(4):499–502. doi: 10.1136/gut.45.4.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen H., Engelbrecht J., Brunak S., von Heijne G. Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng. 1997 Jan;10(1):1–6. doi: 10.1093/protein/10.1.1. [DOI] [PubMed] [Google Scholar]
- Nishiya D., Shimoyama T., Fukuda S., Yoshimura T., Tanaka M., Munakata A. Evaluation of the clinical relevance of the iceA1 gene in patients with Helicobacter pylori infection in Japan. Scand J Gastroenterol. 2000 Jan;35(1):36–39. doi: 10.1080/003655200750024506. [DOI] [PubMed] [Google Scholar]
- Parsonnet J., Friedman G. D., Vandersteen D. P., Chang Y., Vogelman J. H., Orentreich N., Sibley R. K. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991 Oct 17;325(16):1127–1131. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- Peek R. M., Jr, Thompson S. A., Donahue J. P., Tham K. T., Atherton J. C., Blaser M. J., Miller G. G. Adherence to gastric epithelial cells induces expression of a Helicobacter pylori gene, iceA, that is associated with clinical outcome. Proc Assoc Am Physicians. 1998 Nov-Dec;110(6):531–544. [PubMed] [Google Scholar]
- Tomb J. F., White O., Kerlavage A. R., Clayton R. A., Sutton G. G., Fleischmann R. D., Ketchum K. A., Klenk H. P., Gill S., Dougherty B. A. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature. 1997 Aug 7;388(6642):539–547. doi: 10.1038/41483. [DOI] [PubMed] [Google Scholar]
- Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983 Jun 4;1(8336):1273–1275. [PubMed] [Google Scholar]
- Warnich L., Kotze M. J., Groenewald I. M., Groenewald J. Z., van Brakel M. G., van Heerden C. J., de Villiers J. N., van de Ven W. J., Schoenmakers E. F., Taketani S. Identification of three mutations and associated haplotypes in the protoporphyrinogen oxidase gene in South African families with variegate porphyria. Hum Mol Genet. 1996 Jul;5(7):981–984. doi: 10.1093/hmg/5.7.981. [DOI] [PubMed] [Google Scholar]
- Wotherspoon A. C., Ortiz-Hidalgo C., Falzon M. R., Isaacson P. G. Helicobacter pylori-associated gastritis and primary B-cell gastric lymphoma. Lancet. 1991 Nov 9;338(8776):1175–1176. doi: 10.1016/0140-6736(91)92035-z. [DOI] [PubMed] [Google Scholar]
- Yamaoka Y., Kodama T., Gutierrez O., Kim J. G., Kashima K., Graham D. Y. Relationship between Helicobacter pylori iceA, cagA, and vacA status and clinical outcome: studies in four different countries. J Clin Microbiol. 1999 Jul;37(7):2274–2279. doi: 10.1128/jcm.37.7.2274-2279.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Doorn L. J., Figueiredo C., Sanna R., Plaisier A., Schneeberger P., de Boer W., Quint W. Clinical relevance of the cagA, vacA, and iceA status of Helicobacter pylori. Gastroenterology. 1998 Jul;115(1):58–66. doi: 10.1016/s0016-5085(98)70365-8. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Membrane protein structure prediction. Hydrophobicity analysis and the positive-inside rule. J Mol Biol. 1992 May 20;225(2):487–494. doi: 10.1016/0022-2836(92)90934-c. [DOI] [PubMed] [Google Scholar]