Abstract
BACKGROUND—Measurement of 6-thioguanine nucleotide concentrations may be useful for optimising treatment with azathioprine and 6-mercaptopurine. METHODS—We conducted a study of 170 patients with inflammatory bowel disease treated with azathioprine or 6-mercaptopurine to determine the relationship between 6-thioguanine nucleotide concentrations and both disease activity, as measured by the inflammatory bowel disease questionnaire (active disease <170, remission ⩾170) and leucopenia. Blood was submitted for whole blood 6-thioguanine nucleotide concentration and leucocyte count. RESULTS—Mean (SD) inflammatory bowel disease questionnaire score was 176 (32). There was no correlation between inflammatory bowel disease questionnaire scores and 6-thioguanine nucleotide concentrations (rs=−0.09, p=0.24). Median 6-thioguanine nucleotide concentrations in 56 patients with active disease and 114 patients in remission were similar (139 v 131 pmol/8×108 red blood cells; p=0.26). There was no correlation between 6-thioguanine nucleotide concentrations and leucocyte counts. CONCLUSIONS—In patients with inflammatory bowel disease treated with azathioprine or 6-mercaptopurine, 6-thioguanine nucleotide concentrations did not correlate with disease activity, as measured by the inflammatory bowel disease questionnaire, or leucocyte count. These findings are discrepant with most previous studies, possibly due to selection of responding patients who tolerated the medications. A prospective, randomised, dose optimisation trial using 6-thioguanine nucleotide concentrations is warranted. Keywords: azathioprine; 6-mercaptopurine; thioguanine nucleotides; thiopurine methyltransferase; inflammatory bowel disease
Full Text
The Full Text of this article is available as a PDF (154.5 KB).
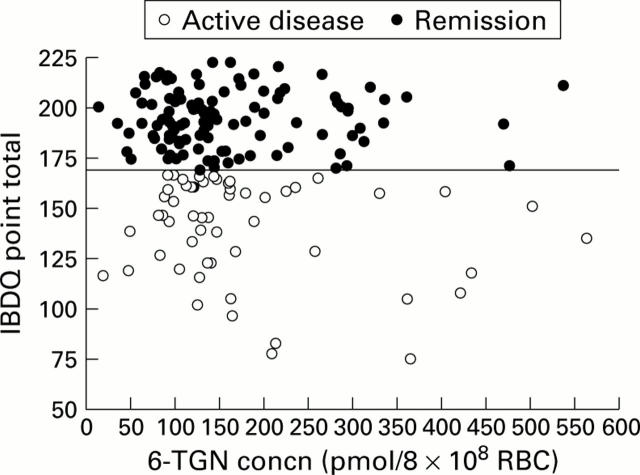
Figure 1 .
Relationship between inflammatory bowel disease questionnaire (IBDQ) score and red blood cell (RBC) 6-thioguanine nucleotide (6-TGN) concentration in patients with active disease and in those in remission.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Awni W. M., Braeckman R. A., Locke C. S., Dubé L. M., Granneman G. R. The influence of multiple oral doses of zileuton on the steady-state pharmacokinetics of sulfasalazine and its metabolites, sulfapyridine and N-acetylsulfapyridine. Clin Pharmacokinet. 1995;29 (Suppl 2):98–104. doi: 10.2165/00003088-199500292-00014. [DOI] [PubMed] [Google Scholar]
- Bergan S., Bentdal O., Sødal G., Brun A., Rugstad H. E., Stokke O. Patterns of azathioprine metabolites in neutrophils, lymphocytes, reticulocytes, and erythrocytes: relevance to toxicity and monitoring in recipients of renal allografts. Ther Drug Monit. 1997 Oct;19(5):502–509. doi: 10.1097/00007691-199710000-00003. [DOI] [PubMed] [Google Scholar]
- Cuffari C., Théorêt Y., Latour S., Seidman G. 6-Mercaptopurine metabolism in Crohn's disease: correlation with efficacy and toxicity. Gut. 1996 Sep;39(3):401–406. doi: 10.1136/gut.39.3.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vos M., Verdievel H., Schoonjans R., Beke R., De Weerdt G. A., Barbier F. High-performance liquid chromatographic assay for the determination of 5-aminosalicylic acid and acetyl-5-aminosalicylic acid concentrations in endoscopic intestinal biopsy in humans. J Chromatogr. 1991 Mar 8;564(1):296–302. doi: 10.1016/0378-4347(91)80094-s. [DOI] [PubMed] [Google Scholar]
- Dubinsky M. C., Lamothe S., Yang H. Y., Targan S. R., Sinnett D., Théorêt Y., Seidman E. G. Pharmacogenomics and metabolite measurement for 6-mercaptopurine therapy in inflammatory bowel disease. Gastroenterology. 2000 Apr;118(4):705–713. doi: 10.1016/s0016-5085(00)70140-5. [DOI] [PubMed] [Google Scholar]
- Erdmann G. R., France L. A., Bostrom B. C., Canafax D. M. A reversed phase high performance liquid chromatography approach in determining total red blood cell concentrations of 6-thioguanine, 6-mercaptopurine, methylthioguanine, and methylmercaptopurine in a patient receiving thiopurine therapy. Biomed Chromatogr. 1990 Mar;4(2):47–51. doi: 10.1002/bmc.1130040202. [DOI] [PubMed] [Google Scholar]
- George J., Present D. H., Pou R., Bodian C., Rubin P. H. The long-term outcome of ulcerative colitis treated with 6-mercaptopurine. Am J Gastroenterol. 1996 Sep;91(9):1711–1714. [PubMed] [Google Scholar]
- Irvine E. J., Feagan B., Rochon J., Archambault A., Fedorak R. N., Groll A., Kinnear D., Saibil F., McDonald J. W. Quality of life: a valid and reliable measure of therapeutic efficacy in the treatment of inflammatory bowel disease. Canadian Crohn's Relapse Prevention Trial Study Group. Gastroenterology. 1994 Feb;106(2):287–296. doi: 10.1016/0016-5085(94)90585-1. [DOI] [PubMed] [Google Scholar]
- Lennard L., Maddocks J. L. Assay of 6-thioguanine nucleotide, a major metabolite of azathioprine, 6-mercaptopurine and 6-thioguanine, in human red blood cells. J Pharm Pharmacol. 1983 Jan;35(1):15–18. doi: 10.1111/j.2042-7158.1983.tb04255.x. [DOI] [PubMed] [Google Scholar]
- Lennard L. The clinical pharmacology of 6-mercaptopurine. Eur J Clin Pharmacol. 1992;43(4):329–339. doi: 10.1007/BF02220605. [DOI] [PubMed] [Google Scholar]
- Lennard L., Van Loon J. A., Lilleyman J. S., Weinshilboum R. M. Thiopurine pharmacogenetics in leukemia: correlation of erythrocyte thiopurine methyltransferase activity and 6-thioguanine nucleotide concentrations. Clin Pharmacol Ther. 1987 Jan;41(1):18–25. doi: 10.1038/clpt.1987.4. [DOI] [PubMed] [Google Scholar]
- Lewis L. D., Benin A., Szumlanski C. L., Otterness D. M., Lennard L., Weinshilboum R. M., Nierenberg D. W. Olsalazine and 6-mercaptopurine-related bone marrow suppression: a possible drug-drug interaction. Clin Pharmacol Ther. 1997 Oct;62(4):464–475. doi: 10.1016/S0009-9236(97)90125-9. [DOI] [PubMed] [Google Scholar]
- Lilleyman J. S., Lennard L. Mercaptopurine metabolism and risk of relapse in childhood lymphoblastic leukaemia. Lancet. 1994 May 14;343(8907):1188–1190. doi: 10.1016/s0140-6736(94)92400-7. [DOI] [PubMed] [Google Scholar]
- Lowry P. W., Franklin C. L., Weaver A. L., Szumlanski C. L., Mays D. C., Loftus E. V., Tremaine W. J., Lipsky J. J., Weinshilboum R. M., Sandborn W. J. Leucopenia resulting from a drug interaction between azathioprine or 6-mercaptopurine and mesalamine, sulphasalazine, or balsalazide. Gut. 2001 Nov;49(5):656–664. doi: 10.1136/gut.49.5.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry P. W., Szumlanski C. L., Weinshilboum R. M., Sandborn W. J. Balsalazide and azathiprine or 6-mercaptopurine: evidence for a potentially serious drug interaction. Gastroenterology. 1999 Jun;116(6):1505–1506. doi: 10.1016/s0016-5085(99)70524-x. [DOI] [PubMed] [Google Scholar]
- Pearson D. C., May G. R., Fick G. H., Sutherland L. R. Azathioprine and 6-mercaptopurine in Crohn disease. A meta-analysis. Ann Intern Med. 1995 Jul 15;123(2):132–142. doi: 10.7326/0003-4819-123-2-199507150-00009. [DOI] [PubMed] [Google Scholar]
- Sandborn W. J., Tremaine W. J., Wolf D. C., Targan S. R., Sninsky C. A., Sutherland L. R., Hanauer S. B., McDonald J. W., Feagan B. G., Fedorak R. N. Lack of effect of intravenous administration on time to respond to azathioprine for steroid-treated Crohn's disease. North American Azathioprine Study Group. Gastroenterology. 1999 Sep;117(3):527–535. doi: 10.1016/s0016-5085(99)70445-2. [DOI] [PubMed] [Google Scholar]
- Szumlanski C. L., Honchel R., Scott M. C., Weinshilboum R. M. Human liver thiopurine methyltransferase pharmacogenetics: biochemical properties, liver-erythrocyte correlation and presence of isozymes. Pharmacogenetics. 1992 Aug;2(4):148–159. [PubMed] [Google Scholar]
- Szumlanski C. L., Weinshilboum R. M. Sulphasalazine inhibition of thiopurine methyltransferase: possible mechanism for interaction with 6-mercaptopurine and azathioprine. Br J Clin Pharmacol. 1995 Apr;39(4):456–459. doi: 10.1111/j.1365-2125.1995.tb04478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinshilboum R. M., Sladek S. L. Mercaptopurine pharmacogenetics: monogenic inheritance of erythrocyte thiopurine methyltransferase activity. Am J Hum Genet. 1980 Sep;32(5):651–662. [PMC free article] [PubMed] [Google Scholar]
- Yu D. K., Elvin A. T., Morrill B., Eichmeier L. S., Lanman R. C., Lanman M. B., Giesing D. H. Effect of food coadministration on 5-aminosalicylic acid oral suspension bioavailability. Clin Pharmacol Ther. 1990 Jul;48(1):26–33. doi: 10.1038/clpt.1990.113. [DOI] [PubMed] [Google Scholar]