Abstract
BACKGROUND AND AIMS—Gastrointestinal disorders is one of the clinical manifestations of chronic Chagas' disease. The pathogenesis seems to be associated with autonomic dysfunction. Here, we consider the muscarinic cholinoceptor mediated alteration in distal colon function in chagasic megacolon. PATIENTS—Patients were divided into four groups: group I, chronic chagasic patients with megacolon; group II, chronic chagasic patients without megacolon; group III, non-chagasic patients with megacolon; and group IV, normal healthy volunteers (control). METHODS—Binding assay and immunoblot of cholinoceptors from human and rat colon and enzyme immunoassay (ELISA) using a synthetic 24mer peptide corresponding to the second extracellular loop of human M2 muscarinic acetylcholine receptors (mAChR) were used to detect the presence of serum antibodies. The effect of antibodies on basal tone and 3',5'-cyclic monophosphate (cAMP) production of human and rat distal colon strips were also tested. RESULTS—Group I but not the other groups had circulating antibodies capable of interacting with human colon activating M2 mAChR, as they competed with binding of specific radioligand to mAChR and interacted with the second extracellular loop of human M2 mAChR. Moreover, affinity purified anti-M2 peptide IgG from group I, in common with monoclonal antihuman M2 mAChR, recognised bands with a molecular weight corresponding to colon mAChR. This antibody also displayed an agonist-like activity, increasing basal tone and decreasing cAMP accumulation. Both effects were blunted by AF-DX 116 and neutralised by the synthetic peptide. CONCLUSIONS—In chagasic patients with megacolon there are antibodies that can recognise and activate M2 mAChR. The implications of these autoantibodies in the pathogenesis of chagasic megacolon is discussed. Keywords: chagasic megacolon; acetylcholine receptor; antibodies; colon contractility
Full Text
The Full Text of this article is available as a PDF (173.0 KB).
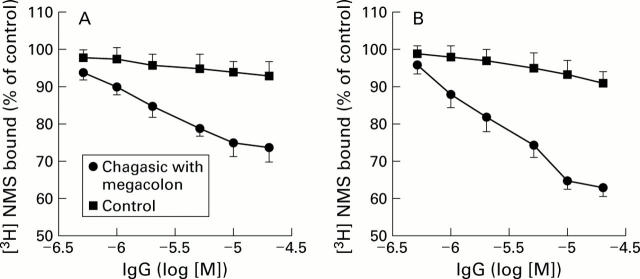
Figure 1 .
Concentration dependent inhibition of [3H] N-methylscopolamine (NMS) binding on colonic smooth muscle membranes. Rat (A) and human (B) colon smooth muscle homogenates were incubated with serum IgG from either chagasic patients with megacolon or normal individuals in the presence of [3H] NMS. Control binding of 100% refers to the binding of the muscarinic radioligand to membranes treated with no IgG. Data are mean (SEM) of triplicate determinations of five IgGs from different chagasic or normal subjects.
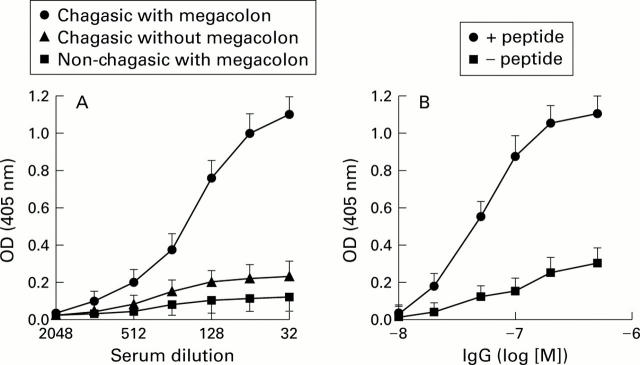
Figure 2 .
Immunoreactivity of anti-muscarinic acetylcholine receptor (mAChR) antibodies from chagasic sera against the second extracellular loop of the human M2 mAChR tested by ELISA. Microtitre wells were coated with 1 µg of M2 peptide and an enzyme immunoassay was performed (see materials and methods). (A) Mean (SEM) optical density (OD) values, representing triplicate assays from 15 chagasic patients with megacolon, 17 chagasic patients without megacolon, and three non-chagasic patients with megacolon. (B) Mean (SEM) optical density (OD) values, representing triplicate determinations of five affinity purified antipeptide IgGs from different chagasic patients with megacolon in the presence (+) or absence (−) of 1×10−5 M peptide.
Figure 3 .
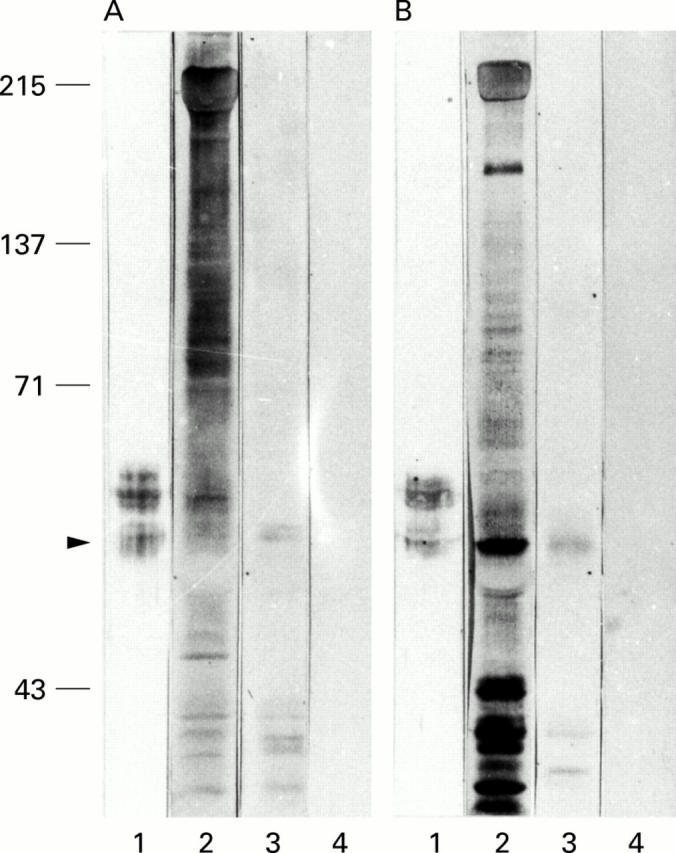
Immunoblotting of M2 muscarinic acetylcholine receptor (mAChR) on rat (A) and human (B) colon smooth muscle homogenates. Lane 2: IgG fraction from chagasic patients with megacolon; lane 3: affinity purified antipeptide IgG from chagasic patients with megacolon; lane 1: monospecific mouse antihuman M2 mAChR IgG; and lane 4: serum IgG fraction from normal patients.
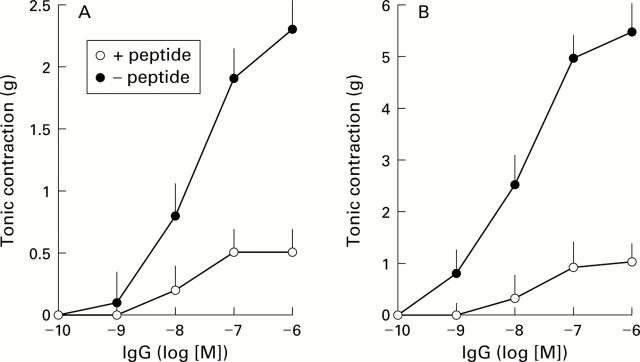
Figure 4 .
Concentration dependent effect of the anti-M2 peptide IgG fraction from chagasic patients with megacolon on rat (A) and human (B) colon tonic contractions. Isolated distal colonic strips were incubated with affinity purified antipeptide antibodies in the presence (+) or absence (−) of 1×10-5 M peptide and tonic contractions were recorded. Values are mean (SEM) of five antipeptide IgG fractions in each group.
Figure 5 .
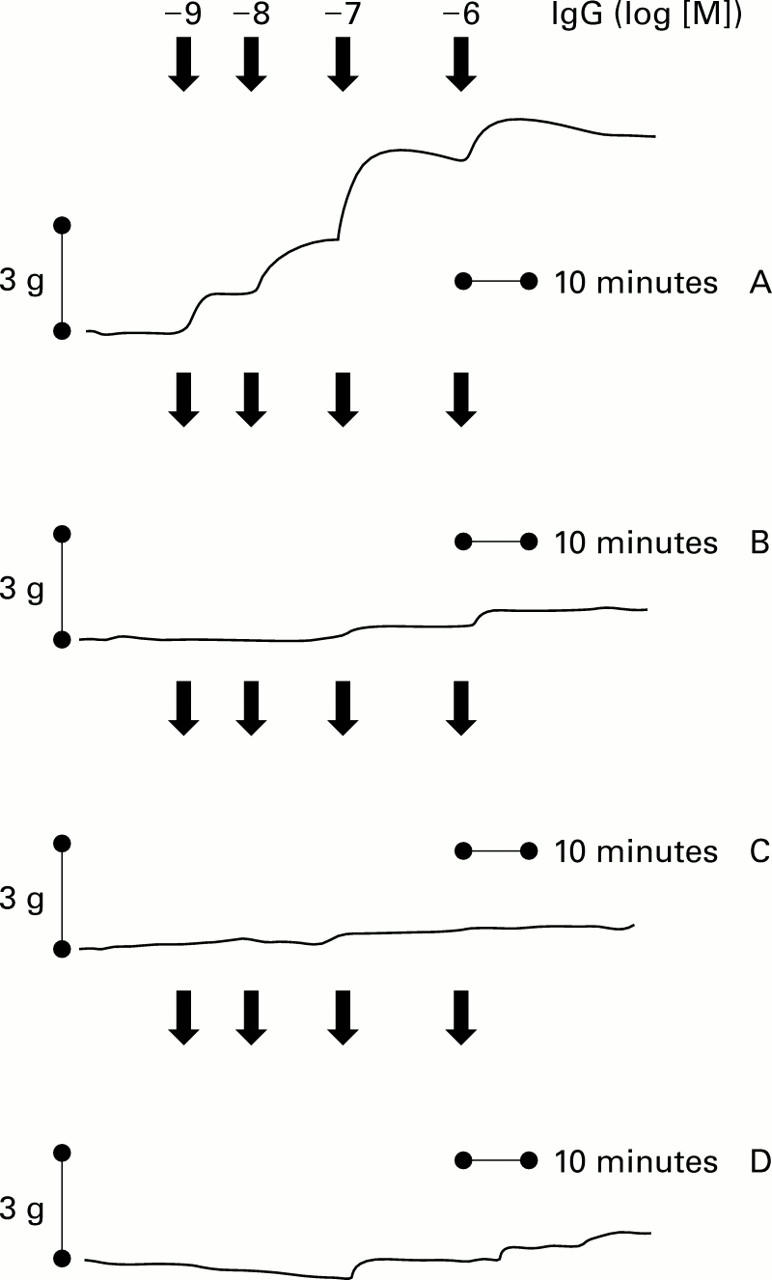
Original tracings showing dose dependent increases in human colon tonic contractions by anti-M2 peptide IgG (IgG log [M]) from chagasic patients with megacolon alone (A) and after exposed tissues to 5×10−6 M AF-DX 116 (B), 5×10−5 M synthetic M2 peptide (C), and 5×10−6 M pertussis toxin (D) over 30 minutes before anti-M2 peptide IgG from chagasic patients with megacolon was added.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blanquet F., Abysique A., Gonella J. In vivo study of the role of muscarinic receptors in the parasympathetic control of rabbit colonic motility. J Auton Nerv Syst. 1994 Mar;46(3):217–227. doi: 10.1016/0165-1838(94)90039-6. [DOI] [PubMed] [Google Scholar]
- Galligan J. J., LePard K. J., Schneider D. A., Zhou X. Multiple mechanisms of fast excitatory synaptic transmission in the enteric nervous system. J Auton Nerv Syst. 2000 Jul 3;81(1-3):97–103. doi: 10.1016/s0165-1838(00)00130-2. [DOI] [PubMed] [Google Scholar]
- Gama A. H., Raia A., Netto A. C. Motility of the sigmoid colon and rectum: contribution to the physiopathology of megacolon in Chagas' disease. Dis Colon Rectum. 1971 Jul-Aug;14(4):291–304. doi: 10.1007/BF02553203. [DOI] [PubMed] [Google Scholar]
- Goin J. C., Borda E., Leiros C. P., Storino R., Sterin-Borda L. Identification of antibodies with muscarinic cholinergic activity in human Chagas' disease: pathological implications. J Auton Nerv Syst. 1994 Apr;47(1-2):45–52. doi: 10.1016/0165-1838(94)90064-7. [DOI] [PubMed] [Google Scholar]
- Goin J. C., Leiros C. P., Borda E., Sterin-Borda L. Interaction of human chagasic IgG with the second extracellular loop of the human heart muscarinic acetylcholine receptor: functional and pathological implications. FASEB J. 1997 Jan;11(1):77–83. doi: 10.1096/fasebj.11.1.9034169. [DOI] [PubMed] [Google Scholar]
- Gómez A., Martos F., Bellido I., Marquez E., Garcia A. J., Pavia J., Sanchez de la Cuesta F. Muscarinic receptor subtypes in human and rat colon smooth muscle. Biochem Pharmacol. 1992 Jun 9;43(11):2413–2419. doi: 10.1016/0006-2952(92)90321-9. [DOI] [PubMed] [Google Scholar]
- Gómez A., Martos F., Bellido I., Marquez E., Garcia A. J., Pavia J., Sanchez de la Cuesta F. Muscarinic receptor subtypes in human and rat colon smooth muscle. Biochem Pharmacol. 1992 Jun 9;43(11):2413–2419. doi: 10.1016/0006-2952(92)90321-9. [DOI] [PubMed] [Google Scholar]
- KOBERLE F. Megaesophagus. Gastroenterology. 1958 Mar;34(3):460–466. [PubMed] [Google Scholar]
- Kunze W. A., Furness J. B. The enteric nervous system and regulation of intestinal motility. Annu Rev Physiol. 1999;61:117–142. doi: 10.1146/annurev.physiol.61.1.117. [DOI] [PubMed] [Google Scholar]
- Köberle F. Chagas' disease and Chagas' syndromes: the pathology of American trypanosomiasis. Adv Parasitol. 1968;6:63–116. doi: 10.1016/s0065-308x(08)60472-8. [DOI] [PubMed] [Google Scholar]
- Leiros C. P., Sterin-Borda L., Borda E. S., Goin J. C., Hosey M. M. Desensitization and sequestration of human m2 muscarinic acetylcholine receptors by autoantibodies from patients with Chagas' disease. J Biol Chem. 1997 May 16;272(20):12989–12993. doi: 10.1074/jbc.272.20.12989. [DOI] [PubMed] [Google Scholar]
- Mathias C. J. Autonomic disorders and their recognition. N Engl J Med. 1997 Mar 6;336(10):721–724. doi: 10.1056/NEJM199703063361010. [DOI] [PubMed] [Google Scholar]
- Meneghelli U. G. Chagas' disease: a model of denervation in the study of digestive tract motility. Braz J Med Biol Res. 1985;18(3):255–264. [PubMed] [Google Scholar]
- Meneghelli U. G., Godoy R. A., Oliveira R. B., Santos J. C., Jr, Dantas R. O., Troncon L. E. Effect of pentagastrin on the motor activity of the dilated and nondilated sigmoid and rectum in Chagas' disease. Digestion. 1983;27(3):152–158. doi: 10.1159/000198945. [DOI] [PubMed] [Google Scholar]
- Meneghelli U. G., de Godoy R. A., Macedo J. F., de Oliveira R. B., Troncon L. E., Dantas R. O. Basal motility of dilated and non-dilated sigmoid colon and rectum in Chagas' disease. Arq Gastroenterol. 1982 Jul-Sep;19(3):127–132. [PubMed] [Google Scholar]
- Ostrom R. S., Ehlert F. J. M2 muscarinic receptor inhibition of agonist-induced cyclic adenosine monophosphate accumulation and relaxation in the guinea pig ileum. J Pharmacol Exp Ther. 1997 Jan;280(1):189–199. [PubMed] [Google Scholar]
- Paaerez Leirós C., Sterin-Borda L., Cossio P., Borda E. S. Muscarinic cholinergic antibody in experimental autoimmune myocarditis regulates cardiac function. Proc Soc Exp Biol Med. 1990 Dec;195(3):356–363. doi: 10.3181/00379727-195-43155. [DOI] [PubMed] [Google Scholar]
- Padovan W., Godoy R. A., Dantas R. O., Meneghelli U. G., Oliveira R. B., Troncon L. E. Lower oesophageal sphincter response to pentagastrin in chagasic patients with megaoesophagus and megacolon. Gut. 1980 Feb;21(2):85–90. doi: 10.1136/gut.21.2.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer G. W., Ehlert F. J. Contractile roles of the M2 and M3 muscarinic receptors in the guinea pig colon. J Pharmacol Exp Ther. 1998 Jan;284(1):269–277. [PubMed] [Google Scholar]
- Sterin-Borda L., Gorelik G., Postan M., Gonzalez Cappa S., Borda E. Alterations in cardiac beta-adrenergic receptors in chagasic mice and their association with circulating beta-adrenoceptor-related autoantibodies. Cardiovasc Res. 1999 Jan;41(1):116–125. doi: 10.1016/s0008-6363(98)00225-9. [DOI] [PubMed] [Google Scholar]
- VIEIRA C. B., DE GODOY R. A., CARRIL C. F. HYPERSENSITIVITY OF THE LARGE INTESTINE TO CHOLINERGIC AGENTS IN PATIENTS WITH CHAGAS' DISEASE AND MEGACOLON. Rev Bras Gastroenterol. 1964 Mar-Apr;16:41–48. [PubMed] [Google Scholar]
- Zhang L. B., Buxton I. L. Muscarinic receptors in canine colonic circular smooth muscle. II. Signal transduction pathways. Mol Pharmacol. 1991 Dec;40(6):952–959. [PubMed] [Google Scholar]